Method for producing organosilicon compounds which have amino groups
An aminoalkyl, organosiloxane technology, applied in organic chemistry, chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, etc., can solve problems such as poor physical properties, visual transparency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
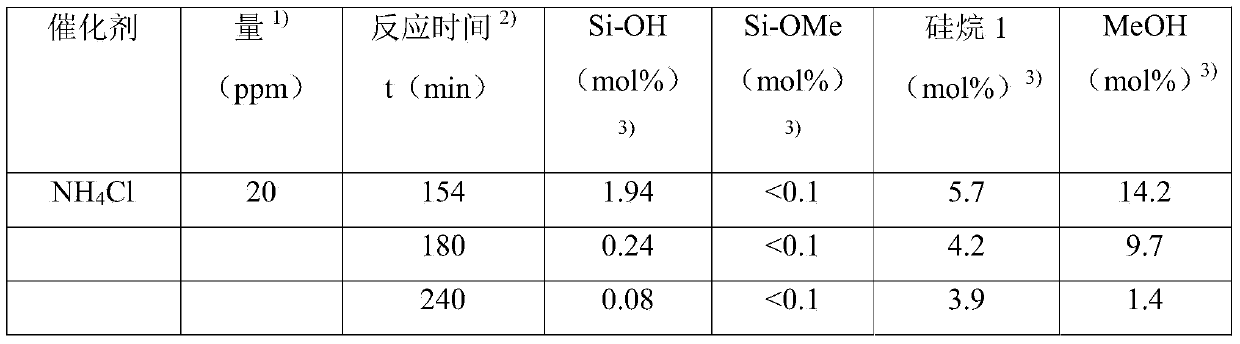
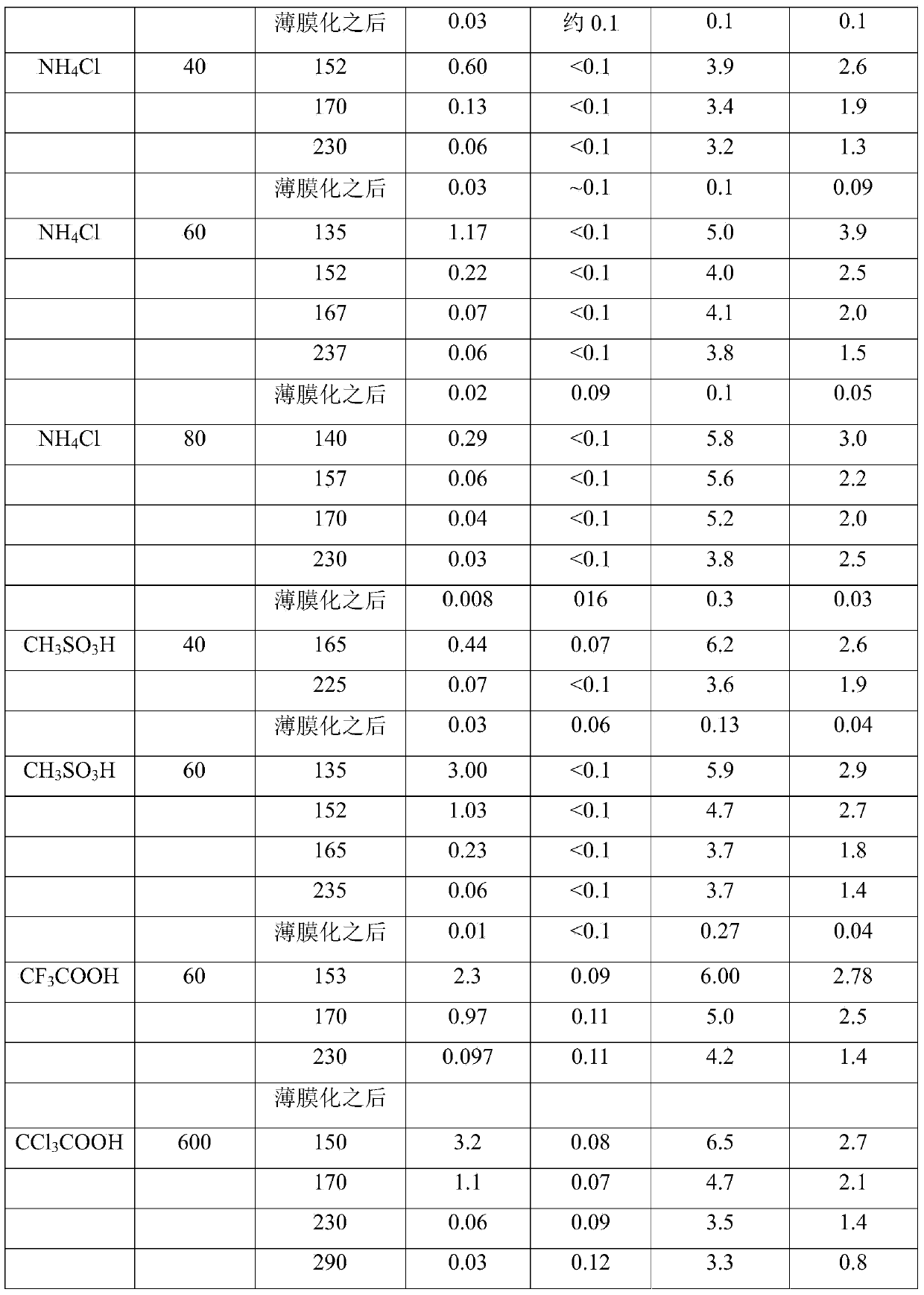
[0089] 330g pass 1 H NMR spectroscopy has 2320g / mol of M n Α,ω-dihydroxy-terminated polydimethylsiloxane (corresponding to 284mmol of Si-OH group) and 250ppm (corresponding to 4.6mmol) determined by Karl Fischer titration The water content is mixed with 6 mg of ammonium chloride and heated to 108-112°C. At this temperature and pressure of about 100mbar, 46.01g (based on Si-OH, 305mmol, corresponding to 1.07 equivalent) of 97.5% 3-aminopropyldimethylmethoxysilane 1 was added within 18min, and received after cooling The methanol formed as a distillate is collected in the vessel. After a total reaction time of 2 hours, the α,ω-diaminopropyl terminated polydimethylsiloxane reaction product was analyzed by NMR spectroscopy. All are based on the molar amount of aminopropyl end groups, with 0.2% Si-OH end groups, 0.06% Si-OMe groups, 2.3% methanol and 3.7% silane 1 detected.
[0090] The product obtained was 10.1 g of a distillate consisting of 97.6% methanol and a mixture of Silane 1...
Embodiment 2
[0093] Example 1 was repeated using 3 mg of ammonium chloride and 46.1 g (306 mmol, 1.08 equivalents) of 97.6% silane 1. The total reaction time is 3 hours.
[0094] At the end of the reaction, based on the molar amount of aminopropyl end groups, the reaction product includes 0.2% Si-OH end groups, 2.2% methanol and 3.2% silane 1. No Si-OMe end groups were detected (the detection limit is about 0.1%).
[0095] The product obtained was 9.8 g of a distillate consisting of 98% methanol and a mixture of silane 1 and 2.0% (0.20 g) cyclic siloxane.
[0096] The pressure was reduced to about 1 mbar to remove excess Silane 1 and methanol from the product.
Embodiment 3
[0098] 330g pass 1 H NMR spectroscopy has 2320g / mol of M n Α,ω-dihydroxy-terminated polydimethylsiloxane (corresponding to 284mmol of Si-OH group) and a water content of 250ppm (corresponding to 4.6mmol) determined by Karl Fischer titration, 6mg of Ammonium chloride and 46.01 g (based on Si-OH, 305 mmol, corresponding to 1.07 equivalents) of 97.5% 31 were mixed at room temperature and heated to 110° C. with stirring at about 110 mbar, while methanol was distilled to a cooled receiver. The heating time is 22 minutes, and the total reaction time is 2 hours.
[0099] At the end of the reaction, based on the molar amount of aminopropyl end groups, the reaction product includes 0.6% Si-OH end groups, 2.3% methanol and 3.6% silane 1. No Si-OMe end groups were detected (the detection limit is about 0.1%).
[0100] The product obtained was 10.0 g of a distillate consisting of 98% methanol and a mixture of Silane 1 and 2.0% (0.20 g) cyclic siloxane.
[0101] The pressure was reduced to abou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com