Docetaxel nanometer micelle, and preparation method and application thereof
A technology of docetaxel and nano micelles, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, and medical preparations containing active ingredients. To solve the problems of drug application, carrier materials without medicinal specifications, etc., to achieve the effect of improving tissue distribution and pharmacokinetic characteristics, improving anti-tumor efficacy, and the preparation method is simple and easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
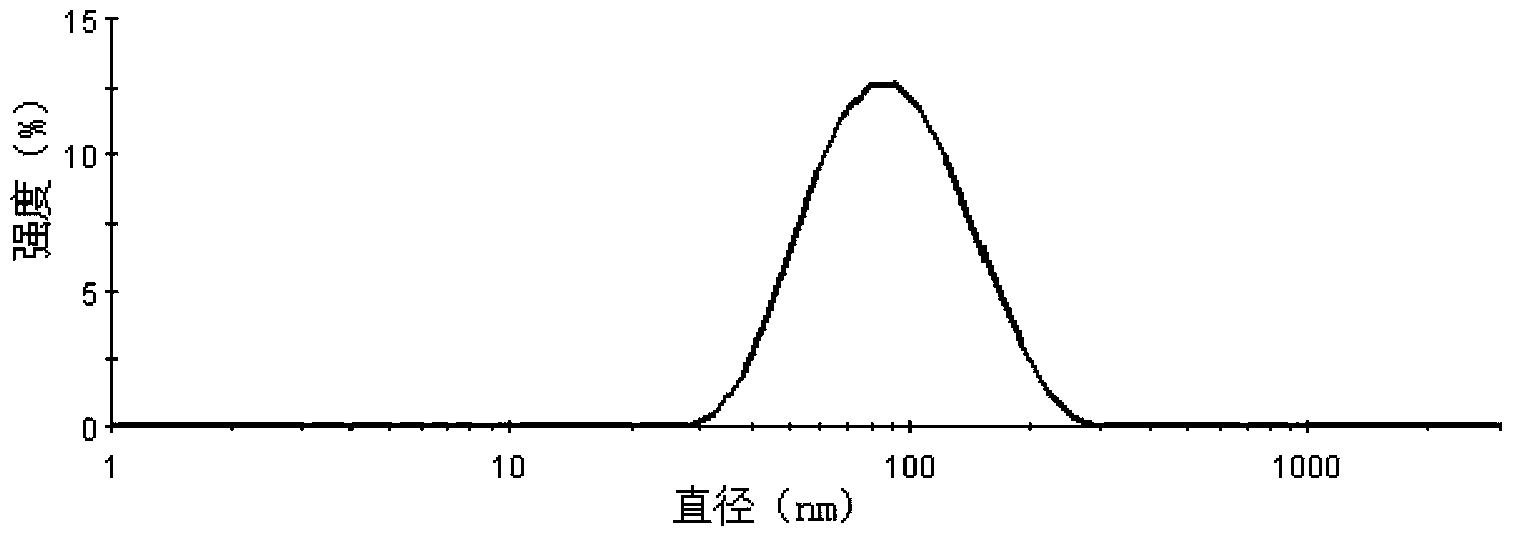
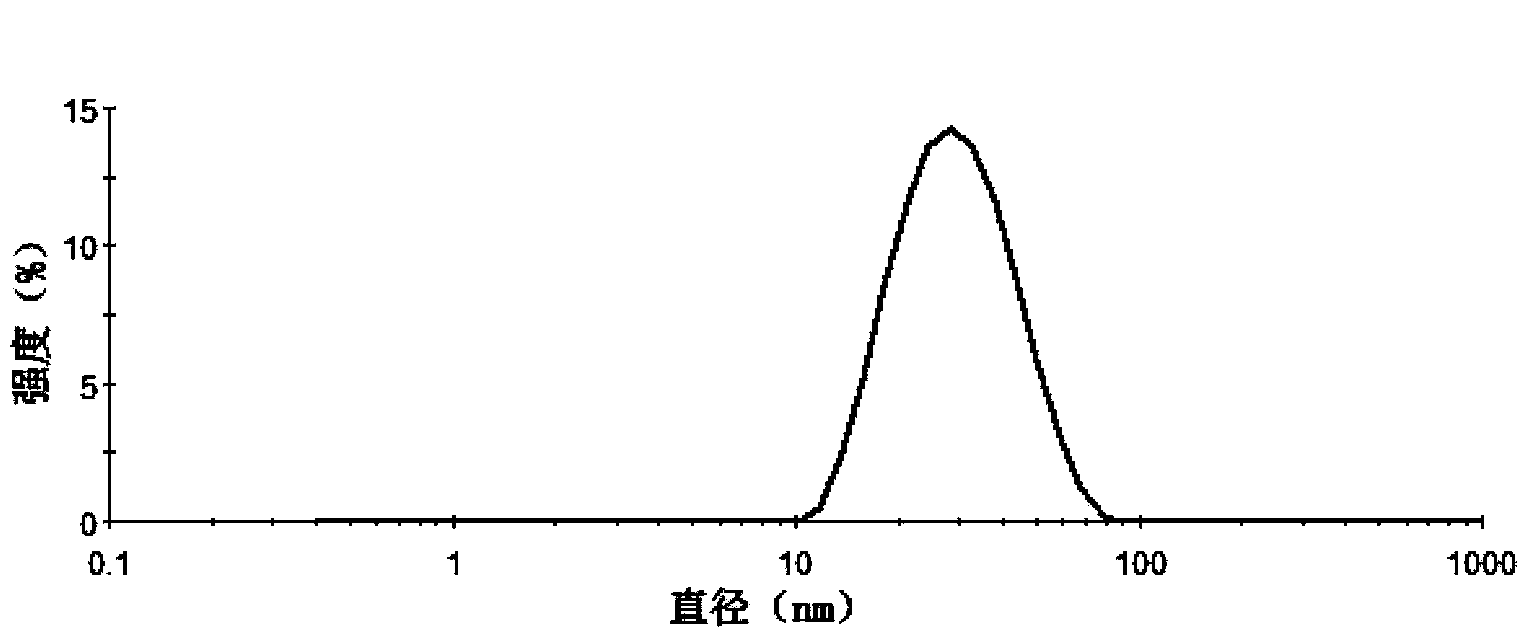
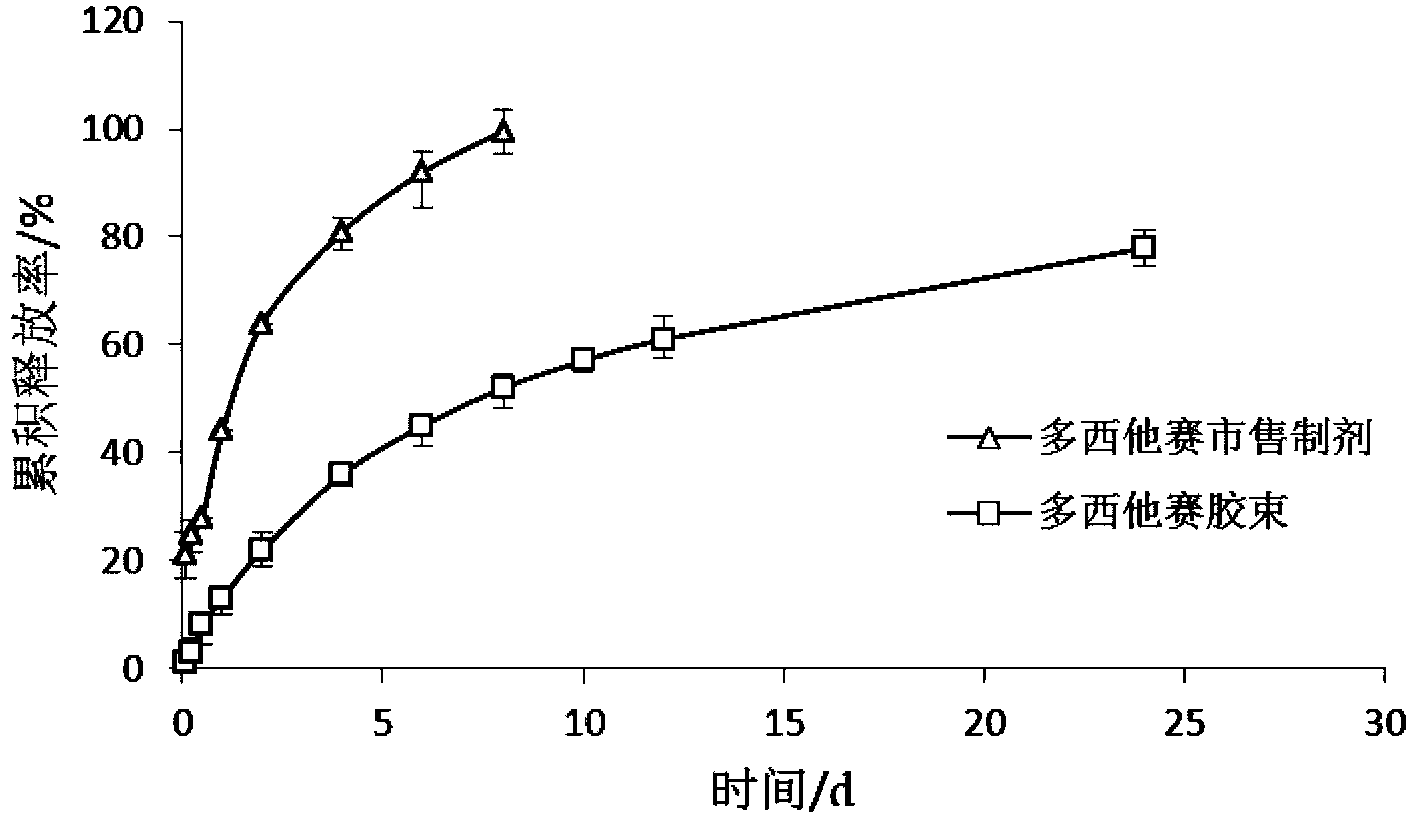
[0049] Weigh 20 mg of docetaxel (purchased from Shanghai Sanwei Pharmaceutical Co., Ltd.), 200 mg of Pluronic L61, 200 mg of PEG-DSPE, and 50 mg of soybean oil, add 0.5 ml of absolute ethanol to dissolve it ultrasonically, and inject it into 5 ml of water (by millipore pure water instrument). (prepared) was stirred at 20,000 rpm at high speed for 1 min, and homogenized under high pressure at 20,000 psi for 5 times to obtain translucent and uniform docetaxel nanomicelles.
Embodiment 2
[0051] Weigh 20 mg of docetaxel, 300 mg of soybean lecithin, 200 mg of Pluronic P85, 200 mg of Pluronic F127, and 50 mg of corn oil, add 0.5 ml of absolute ethanol to dissolve and ultrasonically dissolve them, inject them into 5 ml of pure water and stir at a high speed of 20,000 rpm for 5 min , at 20,000 psi high-pressure homogenization 4 times to obtain translucent and uniform docetaxel nanomicelles.
Embodiment 3
[0053] Weigh 20 mg of docetaxel, HS15800 mg, 400 mg of egg yolk phospholipids, and add 0.2 ml of absolute ethanol to dissolve them, inject them into 5 ml of pure water, stir at a high speed of 15000 rpm for 10 min, and ultrasonicate through the probe (ultrasonic power: 400w , ultrasound time: 10s, ultrasound frequency: 10 times) to obtain docetaxel nanomicelles with translucency and opalescence.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com