A kind of fluorosilicate red fluorescent powder and preparation method thereof
A red phosphor, fluorosilicate technology, applied in chemical instruments and methods, luminescent materials, electrical components, etc., to achieve the effects of strong thermal stability, low temperature, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of Fluorosilicate Red Phosphor Powder Y 3.5 Eu 0.83 (SiO 4 ) 3 f
[0027] (1) Weigh 0.0175 mol of yttrium nitrate and 0.00415 mol of europium nitrate, and dissolve them in deionized water; weigh 0.01 mol of citric acid and 0.0075 mol of ammonium fluoride, respectively, and dissolve them in the above solution.
[0028] (2) Weigh 0.015mol tetraethyl orthosilicate according to the stoichiometric ratio, dissolve it in the solution obtained in (1), and then place it on a magnetic stirrer for heating and stirring; with the evaporation of water, a colloidal precursor is obtained; further heating The precursor burns with a lot of smoke, and after the reaction stops, a fluffy powder is obtained.
[0029] (3) After grinding the obtained powder, calcining in air atmosphere, the calcining temperature is 1100° C., and the calcining time is 2 hours to obtain a fluorosilicate red phosphor.
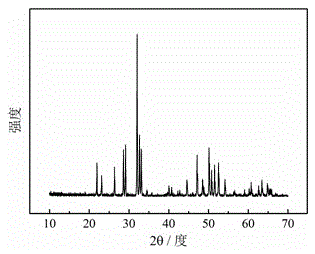
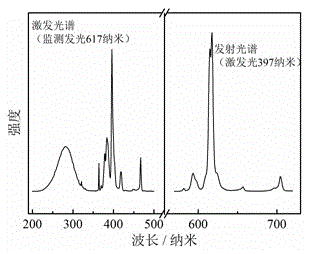
[0030] prepared Y 3.5 Eu 0.83 (SiO 4 ) 3 The X-ray diffraction ...
Embodiment 2
[0031] Example 2 Preparation of Fluorosilicate Red Phosphor Powder Y 4.325 Eu 0.005 (SiO 4 ) 3 f
[0032] (1) Weigh 0.02162 mol of yttrium nitrate and 0.005 mol of europium nitrate, and dissolve them in deionized water; weigh 0.005 mol of citric acid and 0.01 mol of ammonium fluoride, respectively, and dissolve them in the above solutions.
[0033] (2) Weigh 0.015mol tetraethyl orthosilicate according to the stoichiometric ratio, dissolve it in the solution obtained in (1), and then place it on a magnetic stirrer for heating and stirring; with the evaporation of water, a colloidal precursor is obtained; further heating The precursor burns with a lot of smoke, and after the reaction stops, a fluffy powder is obtained.
[0034] (3) After grinding the obtained powder, calcining in air atmosphere, the calcining temperature is 900° C., and the calcining time is 1 hour to obtain a fluorosilicate red phosphor.
Embodiment 3
[0035] Example 3 Preparation of Fluorosilicate Red Phosphor Powder Y 3.13 Eu 1.2 (SiO 4 ) 3 f
[0036] (1) Weigh 0.01565 mol of yttrium nitrate and 0.006 mol of europium nitrate, and dissolve them in deionized water; weigh 0.015 mol of citric acid and 0.005 mol of ammonium fluoride, respectively, and dissolve them in the above solutions.
[0037] (2) Weigh 0.015mol tetraethyl orthosilicate according to the stoichiometric ratio, dissolve it in the solution obtained in (1), and then place it on a magnetic stirrer for heating and stirring; with the evaporation of water, a colloidal precursor is obtained; further heating The precursor burns with a lot of smoke, and after the reaction stops, a fluffy powder is obtained.
[0038] (3) After grinding the obtained powder, calcining in air atmosphere, the calcining temperature is 1000° C., and the calcining time is 5 hours to obtain a fluorosilicate red phosphor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com