Application of sinomenine in the preparation of drugs for the treatment of chronic pain accompanied by depressive behavior
A technology of chronic pain and sinomenine, which is applied in the field of medicine, can solve the problems of low effectiveness, large adverse reactions, and addiction, and achieve the effect of alleviating pain, no sedative side effects, and reducing and improving depression-like behaviors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
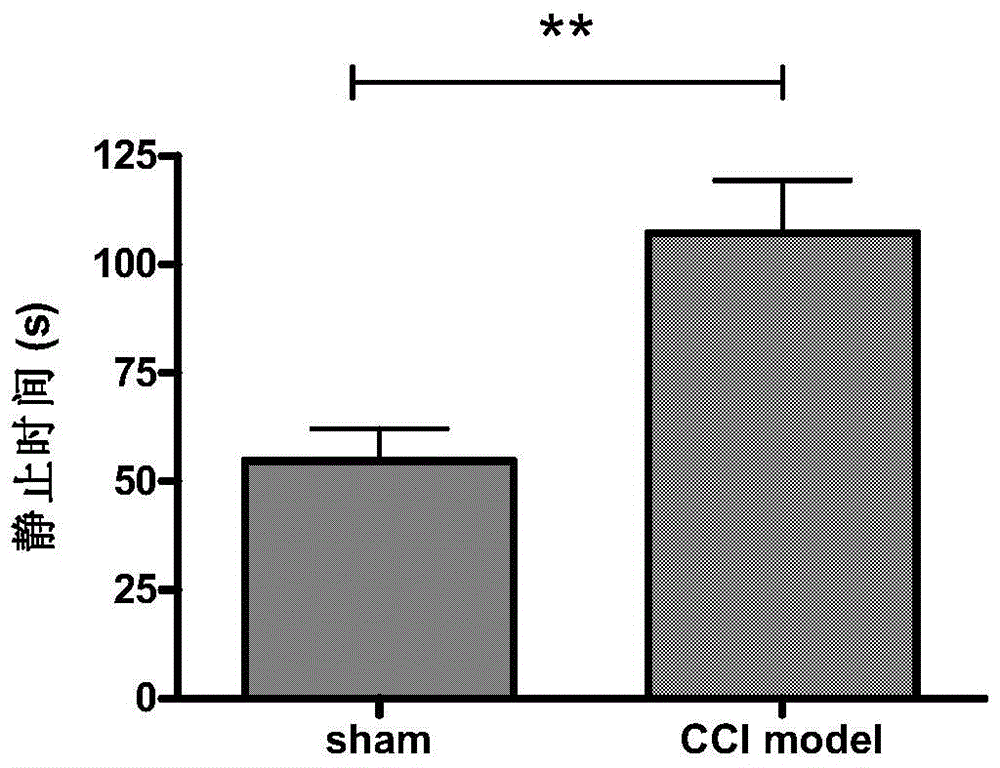
[0035] Example 1: Establishment of a rat model of neuropathic pain-induced depression-like behavior
[0036] 1. Modeling method
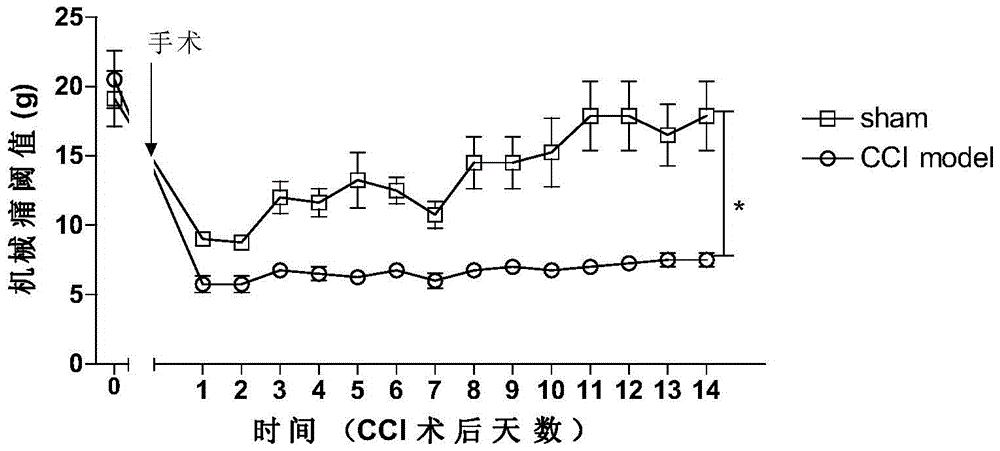
[0037] The establishment method of rat CCI model refers to (Hu B, Doods H, Treede RD, et al. Depression-like behavior in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain, 2009, 143(3): 206-12 .), the specific establishment method:
[0038] Model group operation: after intraperitoneal injection of 7% chloral hydrate anesthesia, surgically expose the right sciatic nerve, at the main part of the nerve, use 4.0 chrome catgut to loosely ligate 4 lines, each catgut is separated by 1-2mm, layer by layer The muscles and skin were sutured, and the rats were fed after operation in a quiet, warm environment avoiding strong light.
[0039] Sham operation group: after anesthetized by intraperitoneal injection of 7% chloral hydrate, the right sciatic nerve was exposed and separated, and the muscles and skin were sutured layer by l...
Embodiment 2
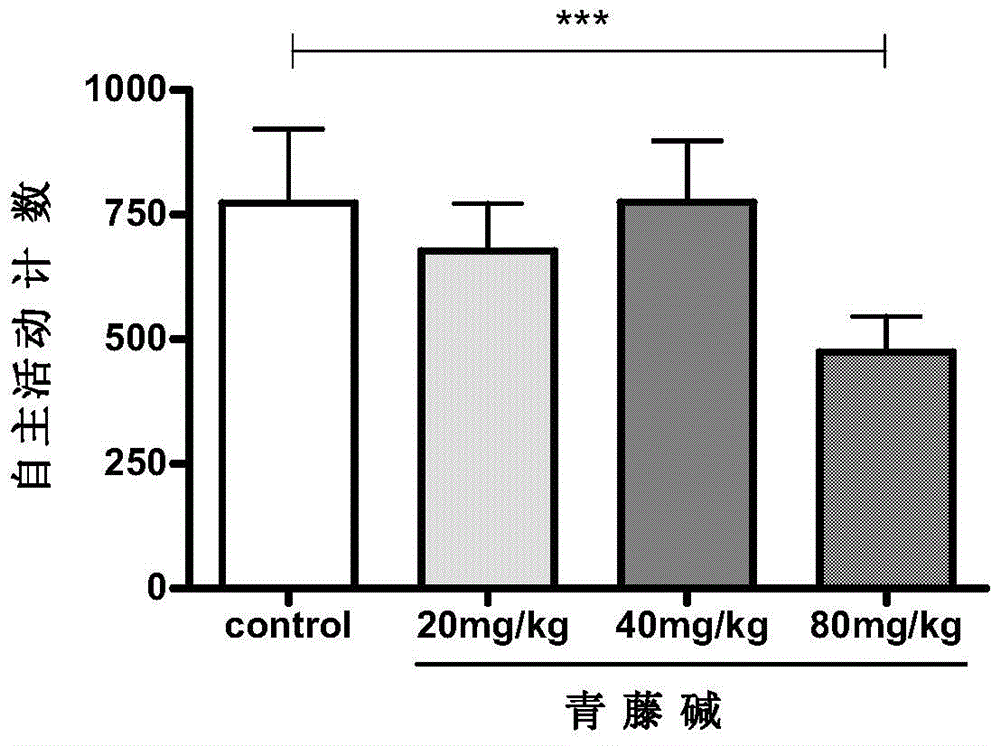
[0051] Embodiment 2: Sinomenine sedation and cumulative sedative effect experiment
[0052] The day before the operation, intraperitoneal injection was administered, and the rats' autonomous activities were measured. Compared with the normal saline group, if there was a sedative effect, the dose was adjusted.
[0053] Sinomenine sedation test:
[0054] 1. Animal grouping:
[0055] 40 male rats; divided into 4 groups, ie normal control group (control) and sinomenine 20mg / kg, 40mg / kg, 80mg / kg three dosage groups, 10 in each group.
[0056] 2. The normal control group was given normal saline, and the other groups were given sinomenine 20mg / kg, 40mg / kg, and 80mg / kg respectively, and observed their spontaneous activities within 1 hour. The results showed that the voluntary activity counts in the 80mg / kg group were significantly lower than those in the normal control group, with a statistical difference (P image 3 ).
[0057] 3. Cumulative sedative effect of sinomenine 40mg / kg: s...
Embodiment 3
[0059] Example 3: Effects of Acute Administration of Sinomenine 10-40 mg / kg on Mechanical Pain Threshold of CCI Model Rats
[0060] Animal grouping: 40 male rats; model group + normal saline (10); model group + sinomenine (10mg / kg) 10; model group + sinomenine (20mg / kg) 10; model group + 10 sinomenine (40mg / kg) (note: administered by intraperitoneal injection, the solvent of sinomenine is normal saline).
[0061] The operation is the same as above, and the basic value of pain is tested on the second day after the operation (did not yet administered at this time). After the basic threshold test is completed, if the pain threshold decreases, the drug is administered by intraperitoneal injection immediately, and at intervals of 30 minutes after administration. Test the pain threshold once until the threshold returns to normal. The method for determining the pain threshold was the same as before. The result is as Figure 5 As shown, 24 hours after CCI, the baseline value of mec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com