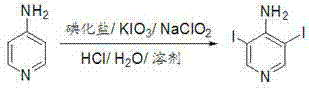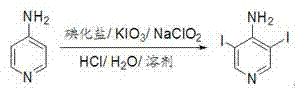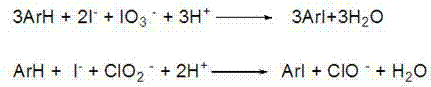Method for synthesizing 3,5-diiodo-4-aminopyridine by employing in-situ iodized reagent production method
A technology of aminopyridine and synthesis method, which is applied in the field of synthesis of iodoaminopyridine, can solve the problems of instability of iodine chloride, easy blockage of condensation pipe, low reaction yield, etc., and achieve good economic and social benefits and process operation Simple process and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In the reaction flask, add 500 mL of methanol, 3000 mL of water, 94.11 g (1.0 mol) of 4-aminopyridine, 224.10 g (1.35 mol) of potassium iodide, and 149.80 g (0.70 mol) of potassium iodate in sequence, and slowly drop them at 20°C Concentrated hydrochloric acid 160mL, it takes 3 hours to add dropwise, continue to stir and react for 1 hour after dropwise addition, then drop into 50mL aqueous solution containing 17.73g (0.20mol) of sodium chlorite, continue to stir and react for 0.5 hour after dropwise addition, high efficiency After the liquid chromatography central control monitors the reaction, the reaction solution is diluted with 4000mL of water, and 6000mL of extractant trichloromethane is added three times, and the combined organic phase is washed once with brine containing sodium thiosulfate and sodium bicarbonate containing Wash once with salt water, separate the phases, dry over anhydrous magnesium sulfate, and remove the solvent chloroform under reduced pressure ...
Embodiment 2
[0033] In the reaction described in Example 1, 201.02 g (1.35 mol) of sodium iodide was substituted for 224.10 g (1.35 mol) of potassium iodide, and other operations were the same as in Example 1.
[0034] According to the operation of Example 1, 303.36 g of white crystalline solid 3,5-diiodo-4-aminopyridine was obtained, with a yield of 87.7% and a relative liquid phase content of 98.3%.
Embodiment 3
[0036] In the reaction described in Example 1, the amount of potassium iodate used was 256.80 g (1.20 mol), and other operations were the same as in Example 1.
[0037]The operation was carried out according to Example 1, and 315.47 g of white crystalline solid 3,5-diiodo-4-aminopyridine was obtained, with a yield of 91.2% and a relative liquid phase content of 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com