Chinese patent medicine tablets for treating cardiovascular and cerebrovascular diseases and preparation method of Chinese patent medicine tablets
A technology of cardiovascular and cerebrovascular diseases and Chinese patent medicines, which is applied in the field of preparation of plant extract pharmaceutical dosage forms, can solve the problems of singleness and insignificant effect, achieve the effect of auxiliary treatment, improve cardiovascular and cerebrovascular diseases, and make up for the limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
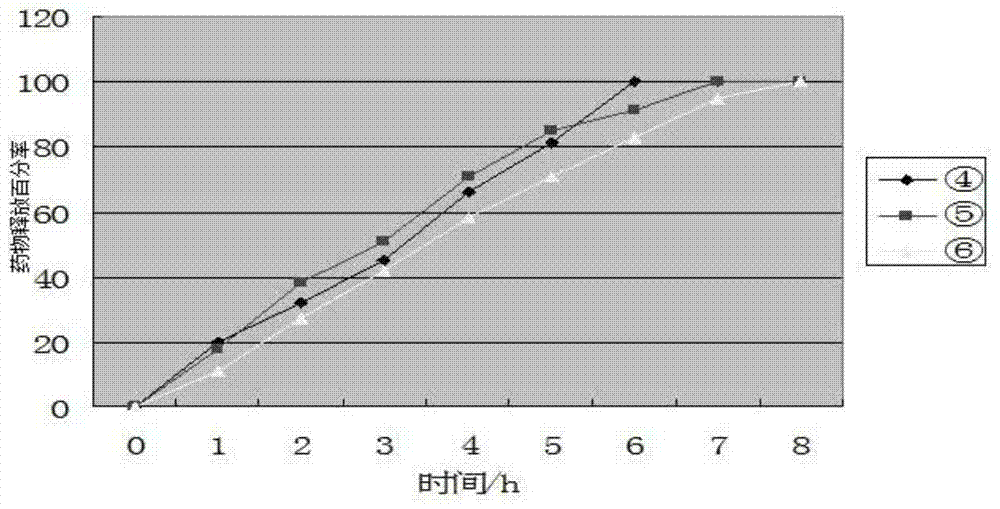
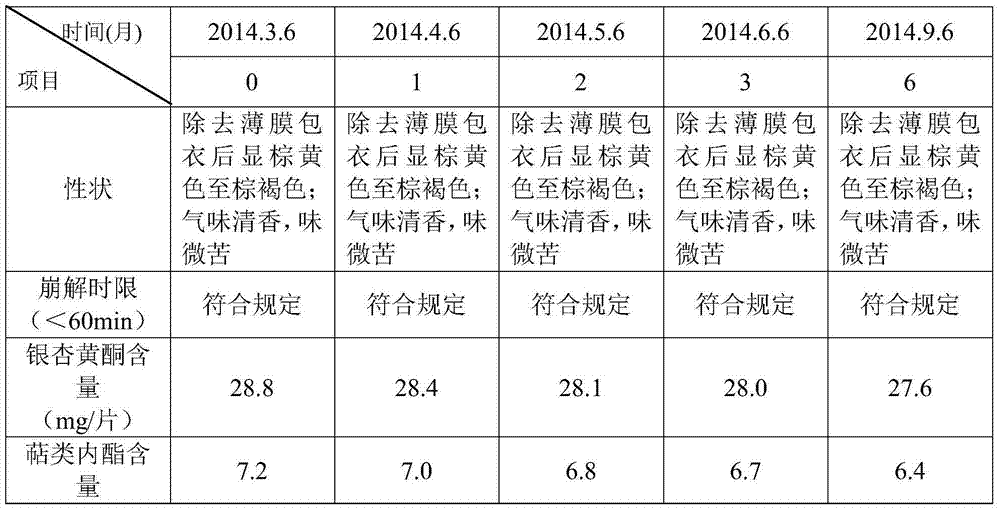
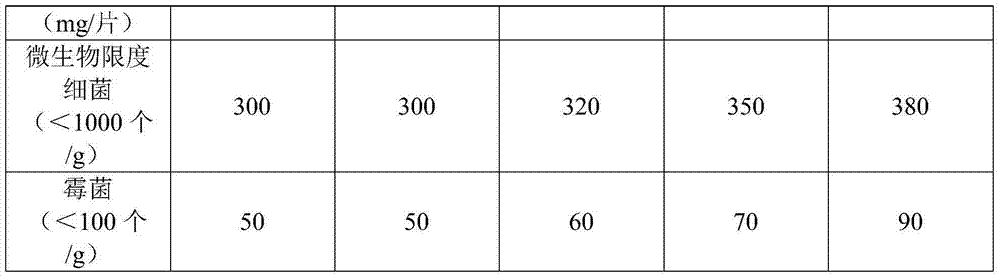
Image
Examples
Embodiment 1
[0029] A Chinese patent medicine tablet for treating cardiovascular and cerebrovascular diseases, the main components and auxiliary materials are:
[0030] 20g Ginkgo biloba extract, 10g Polygonum multiflorum extract, 10g Uncaria extract, 40g carrier PVP-K30, 40g soluble starch, 20g magnesium stearate.
[0031] Ginkgo biloba extract, Polygonum multiflorum extract, and Uncaria extract are crushed and powdered, passed through a 100-mesh sieve, and the remaining materials are respectively passed through a 100-mesh sieve; the three drugs, carriers and soluble starch are mixed evenly in proportion, and an appropriate amount of water is added. Make it into a sticky paste; dry it in an electric constant temperature drying oven, set the temperature at 70°C, and crush it with a pulverizer after the drug is dry, and pass it through a 100-mesh sieve; set the tablet pressing pressure of the tablet machine to 10KN, and the filling depth to 12mm. The thickness of the dose is 4mm; magnesium ...
Embodiment 2
[0033] A Chinese patent medicine tablet for treating cardiovascular and cerebrovascular diseases, the main components and auxiliary materials are:
[0034] 30g Ginkgo biloba extract, 10g Polygonum multiflorum extract, 10g Uncaria extract, 50g carrier PVP-K30, 50g soluble starch, 30g magnesium stearate.
[0035] The preparation method of the present invention comprises: pulverizing the extract of ginkgo biloba, the extract of Polygonum multiflorum, and the extract of Uncaria uncaria into powder, passing through a 100-mesh sieve, and passing the remaining substances through a 100-mesh sieve respectively. Mix the three kinds of medicines, carrier and soluble starch evenly in proportion, add appropriate amount of water to make it sticky. Dry in an electric thermostat oven with a set temperature of 70°C. After the medicine is dry, crush it with a pulverizer and pass through a 100-mesh sieve. Set the tablet pressing pressure of the tablet press to 10KN, the filling depth to 12mm, ...
Embodiment 3
[0037] A Chinese patent medicine tablet for treating cardiovascular and cerebrovascular diseases, the main components and auxiliary materials are:
[0038] 40g Ginkgo biloba extract, 20g Polygonum multiflorum extract, 20g Uncaria extract, 100g carrier PVP-K30, 80g soluble starch, 40g magnesium stearate.
[0039] The preparation method of the present invention comprises: pulverizing the extract of ginkgo biloba, the extract of Polygonum multiflorum, and the extract of Uncaria uncaria into powder, passing through a 100-mesh sieve, and passing the remaining substances through a 100-mesh sieve respectively. Mix the three kinds of medicines, carrier and soluble starch evenly in proportion, add appropriate amount of water to make it sticky. Dry in an electric thermostat oven with a set temperature of 70°C. After the medicine is dry, crush it with a pulverizer and pass through a 100-mesh sieve. Set the tablet pressing pressure of the tablet press to 10KN, the filling depth to 12mm,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com