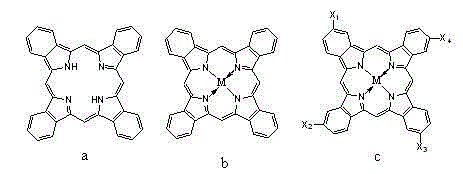
Preparation method of iron phthalocyanine
A technology of iron phthalocyanine and iron salt, which is applied in the field of compound preparation and can solve problems such as health hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (Iron salt: ferrous chloride, solvent: dioctyl phthalate)
[0026] In a 250 mL three-neck flask, add 2 g of iron salt (ferrous chloride, 10 mmol), 4 g of phthalic anhydride (27 mmol), 8.1 g of urea (133 mmol), and 24 mL of dioctyl phthalate in sequence, and feed After completion, heat and stir. When the temperature reaches 130°C, add 0.12 g ammonium molybdate (0.6 mmol) and 0.147 g ammonium chloride (2.7 mmol) and keep the reaction for 2 h until the reactant has no bubbles, then you can proceed to the next step Reaction; the heating process is about 1°C / min, and the temperature is directly raised to 190°C, and the reaction is held for 6 hours. After the reaction, the reaction temperature will be lowered to room temperature; Put it into 100 mL ethanol, stir and heat to 40°C and filter, then put the filter cake into 100 mL water, add 0.5 g sodium dodecylbenzenesulfonate at the same time, heat to 80°C and stir for 30 min, filter and dry to get the product iron phthalocyani...
Embodiment 2
[0028] (iron salt: ferrous sulfate, solvent: recycled dioctyl phthalate)
[0029] In a 250 mL three-necked flask, 1.7 g of iron salt (ferrous sulfate, 10 mmol), 4 g of phthalic anhydride (27 mmol), 8.1 g of urea (133 mmol), and 26 mL of recovered dioctyl phthalate were successively added. After the feeding is completed, heat and stir. When the temperature reaches 120~140°C, put in 0.12 g ammonium molybdate (0.6 mmol) and 0.147 g ammonium chloride (2.7 mmol), and keep it warm for 1 hour until the reactant has no bubbles, then it can be carried out The next step of the reaction: the reaction product of a, the heating process is about 1°C / min, the temperature is directly raised to 180°C, and the temperature is kept for 4 hours. After the reaction, the reaction temperature will be lowered to room temperature; the product is filtered, and the filtrate is recovered. Dioctyl phthalate About 21 mL, put the filter cake into 100 mL ethanol, stir and heat to 40°C for filtration, then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com