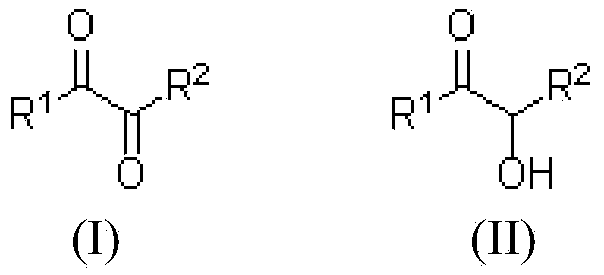Electrochemical catalytic synthesis method of alpha-carbonyl ketone compounds
A synthesis method and compound technology, applied in the field of electrochemical catalytic synthesis of α-carbonyl ketones, can solve the problems of increased reaction cost and post-processing complexity, ineffective reaction, and high-concentration organic waste discharge. Achieve high yield, avoid equipment cost and reduce reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the electrocatalytic synthesis of 2,3-pentanedione
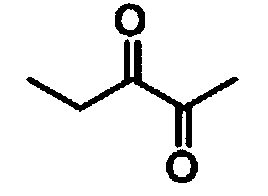
[0027] In a 400 mL single-chamber electrolytic cell, 100 mmol of 2-hydroxy-3-pentanone was dissolved in 200 mL of NaBr (10 mmol) aqueous solution, and 100 mL of dichloromethane was added. Under stirring at room temperature, with graphite as the anode and iron sheet as the cathode, at 30mA / cm 2 electrolysis at a constant current. When the current efficiency reaches 59%, the electrolysis is stopped, the layers are static, and the dichloromethane in the lower organic phase is removed under normal pressure to obtain 2,3-pentanedione with a yield of 78%.
[0028]
[0029] Yellow-green liquid; bp 110-112°C; 1 H NMR (400MHz, CDCl 3 ): δ1.09(t, 3H, J=7.6Hz), 2.17(s, 3H), 2.44(q, 2H, J=7.6Hz).
Embodiment 2
[0030] Example 2: Electrocatalytic synthesis of 2,3-pentanedione
[0031] As in Example 1, 2,3-pentanedione was synthesized by electrocatalysis using 3-hydroxy-2-pentanone as a raw material, with a yield of 76%.
[0032]
[0033] Yellow-green liquid; bp 110-112°C; 1 H NMR (400MHz, CDCl 3 ): δ1.09(t, 3H, J=7.6Hz), 2.17(s, 3H), 2.44(q, 2H, J=7.6Hz).
Embodiment 3
[0034] Example 3: Electrocatalytic synthesis of 3,4-hexanedione
[0035] As in Example 1, 3,4-hexanedione was electrocatalytically synthesized using propioin as a raw material, with a yield of 85%.
[0036]
[0037] Yellow-green liquid; bp 131°C; 1 H NMR (400MHz, CDCl 3 ): δ1.10(t, 3H, J=7.6Hz), 2.47(q, 2H, J=7.6Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com