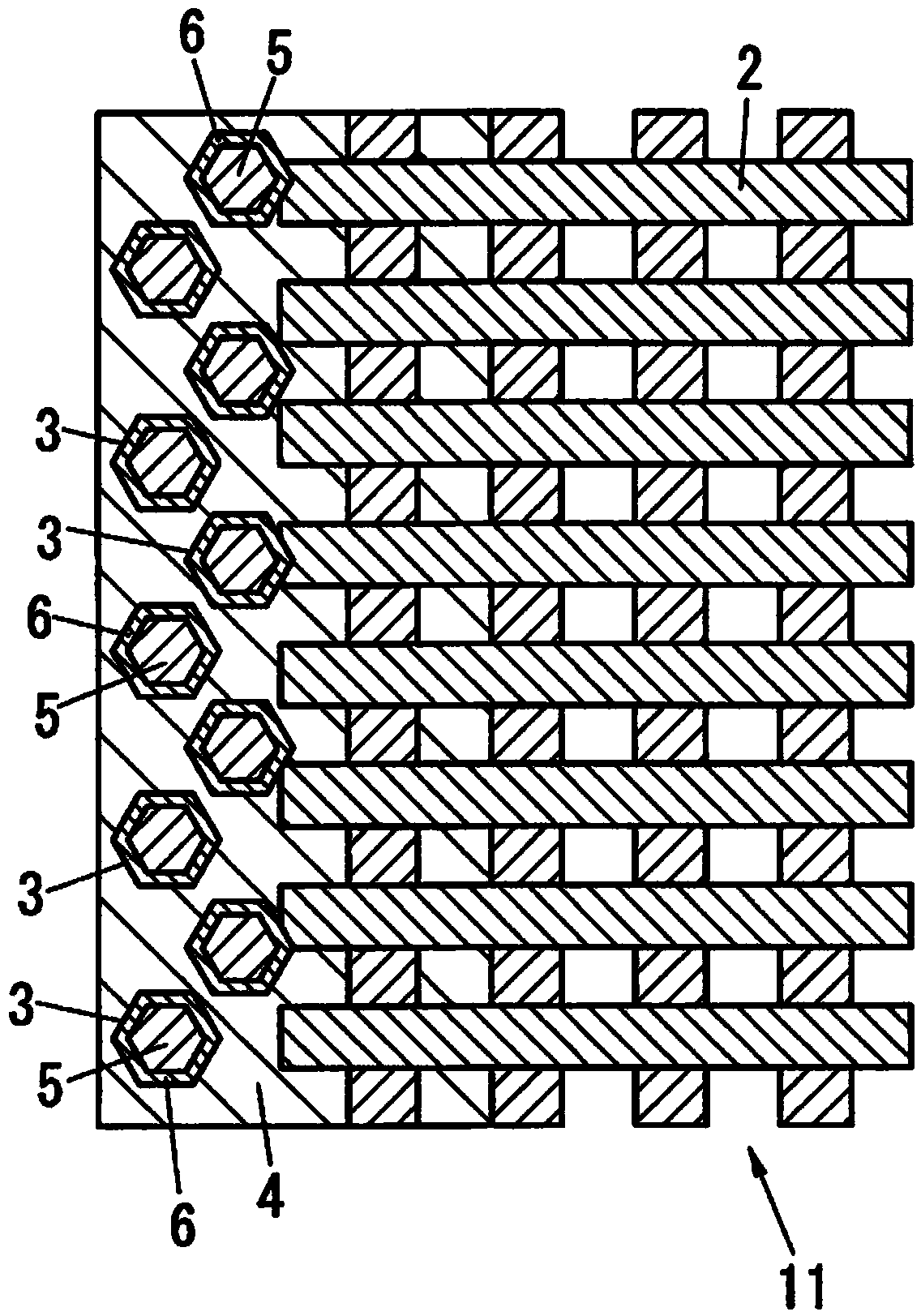
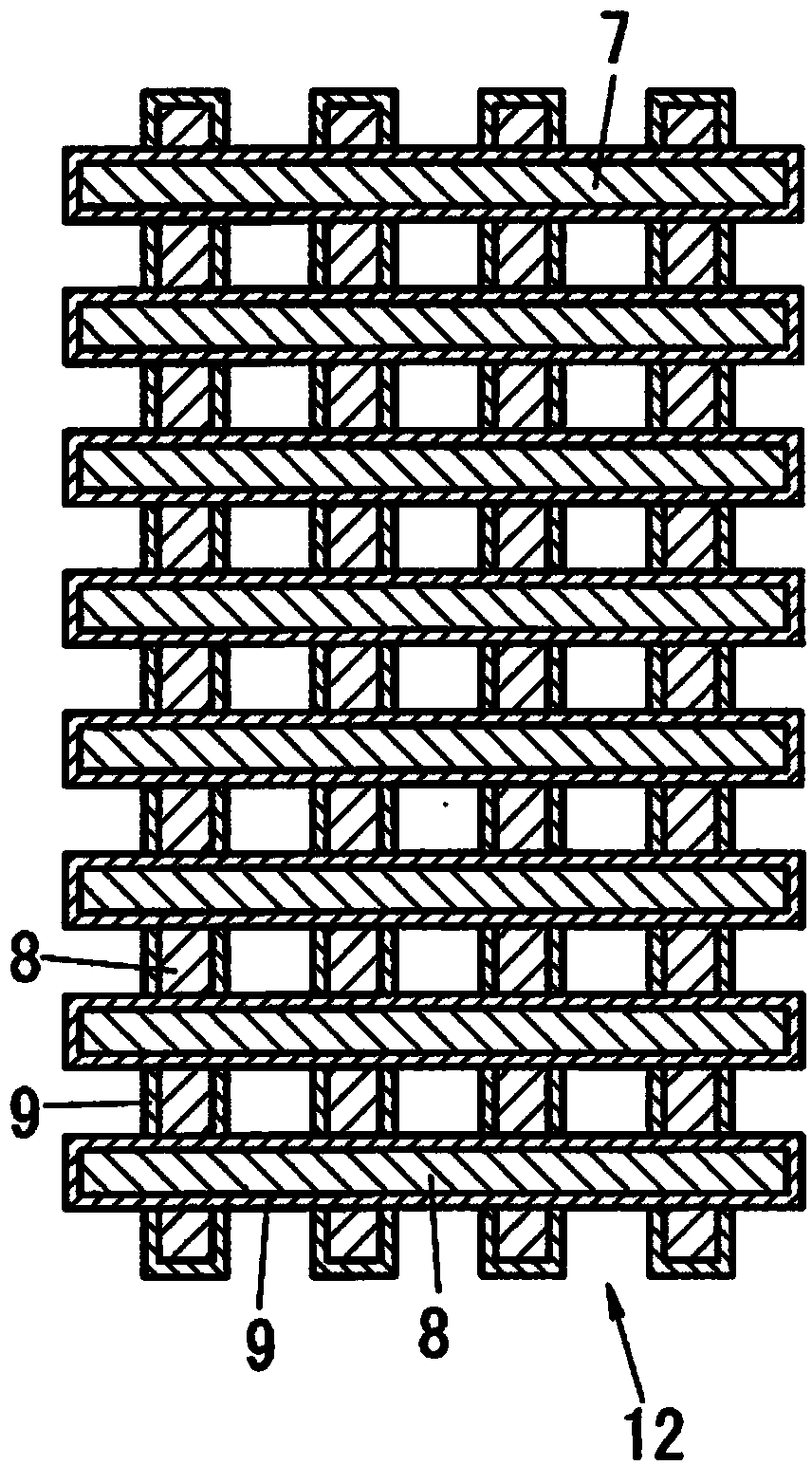
Carbon-based material, electrode catalyst, electrode, gas diffusion electrode, electrochemical device, fuel battery, and process for producing carbon-based material
A technology of carbon-based materials and carbon sources, which is applied in catalyst activation/preparation, solid electrolyte fuel cells, fuel cells, etc., can solve the problems of rare, expensive, and unstable cost of precious metals, and achieve the effect of easy acquisition and improved catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0243] 3 g of graphite in particle shape (median diameter: 6 μm), 0.1 M aqueous solution of iron(III) chloride, and 0.15 M ethanol solution of pentaethylenehexamine were added to a container to prepare a mixture. The amount of the 0.1M aqueous solution of iron(III) chloride used was chosen so that the percentage of iron atoms relative to the graphite was 10% by mass. Ethanol was further added to the mixture so that the total volume of the resulting mixture was 9 mL. The resulting mixture was subjected to ultrasonic dispersion, and then dried at 60° C. using a drier. The result was a sample containing graphite, iron(III) chloride and pentaethylenehexamine.
[0244] The sample thus obtained was placed at the end of a quartz tube, and the atmosphere inside the quartz tube was replaced with argon. The quartz tube was inserted into an oven at 900° C., left for 45 seconds, and then taken out. Adjust the heating rate at the beginning of heating the sample to 300 °C / s by adjusting ...
Embodiment 2
[0247] The carbon-based material in Example 2 was prepared in the same manner and under the same conditions as in Example 1, except that 0.1M cobalt chloride aqueous solution was used instead of ferric chloride as the metal source.
Embodiment 3
[0249] The carbon-based material in Example 3 was prepared in the same manner and under the same conditions as in Example 1, except that 0.1M manganese chloride aqueous solution was used instead of ferric chloride as the metal source.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com