Xanthene fluorescent dye with biofilm permeability potential and preparation method thereof
A technology for fluorescent dyes and xanthenes, which is applied in the field of preparation of xanthenes dyes, and can solve the problems of narrow application range and unreliable photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
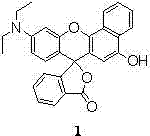
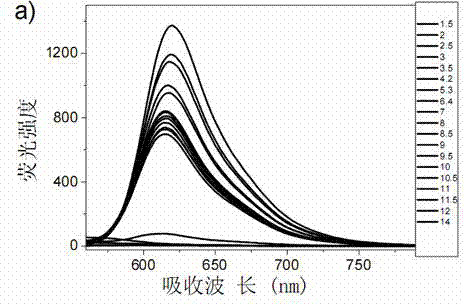
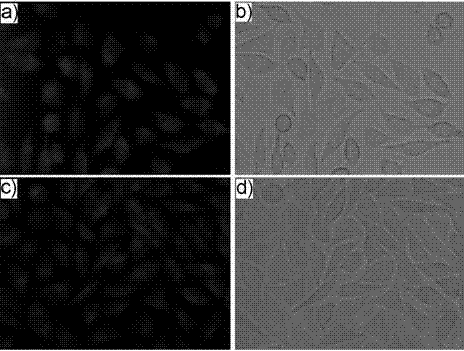
[0031] Synthesis of a novel pH-stable and membrane-permeable xanthene-based dye Compound 1 with potential for biorelevant applications:
[0032]
[0033] Add 3.133 g (10 mmol) of 2-(4-diethylamino)-2-hydroxybenzoyl)benzoic acid and 1.601 g (10 mmol) of 1,4-dihydroxynaphthalene into a 100 mL round bottom flask, stirring 10 ml of methanesulfonic acid was added dropwise, and the reaction was stopped at 85 °C for 4 h. Add 100 mL of water to the reaction solution, add appropriate amount of NaHCO 3 The solid neutralized the reaction solution, extracted with ethyl acetate to collect the organic phase, and washed with anhydrous Na 2 SO 4 After drying and filtering, the obtained solid crude product was recrystallized with 10 mL of methanol solution, and suction filtered to obtain 3.820 g of bright red solid powder, namely compound 1, with a yield of 87%. Melting point: 256 – 258 °C; HRMS (LC / MS) m / z: [M + H] + = 438.1705; 1 H NMR (400 MHz, DMSO-d 6 , ppm) δ 9.93 (S, 1H), 8.54...
Embodiment 2
[0035] Synthesis of a novel pH-stable and membrane-permeable xanthene-based dye Compound 1 with potential for biorelevant applications:
[0036]
[0037] Add 3.102 g (10 mmol) of 2-(4-diethylamino)-2-hydroxybenzoyl)benzoic acid and 1.605 g (10 mmol) of 1,4-dihydroxynaphthalene into a 100 mL round bottom flask, stirring 10 ml of methanesulfonic acid was added dropwise, and the reaction was stopped at 80 °C for 5 h. Add 100 mL of water to the reaction solution, add appropriate amount of NaHCO 3 The solid neutralized the reaction solution, extracted with ethyl acetate to collect the organic phase, and washed with anhydrous Na 2 SO 4 After drying and filtering, the obtained solid crude product was recrystallized with 12 mL of methanol solution, and suction filtered to obtain 3.732 g of bright red solid powder, namely compound 1, with a yield of 85%. Melting point: 256 – 258 °C; HRMS (LC / MS) m / z: [M + H] + = 438.1705; 1 H NMR (400 MHz, DMSO-d 6 , ppm) δ 9.93 (S, 1H), 8.54...
Embodiment 3
[0039] Synthesis of a novel pH-stable and membrane-permeable xanthene-based dye Compound 1 with potential for biorelevant applications:
[0040]
[0041] Add 2.998 g (10 mmol) of 2-(4-diethylamino)-2-hydroxybenzoyl)benzoic acid and 1.601 g (10 mmol) of 1,4-dihydroxynaphthalene into a 100 mL round bottom flask, stirring 10 ml of methanesulfonic acid was added dropwise, and the reaction was stopped at 83 °C for 4 h. Add 120 mL of water to the reaction solution, add appropriate amount of NaHCO 3 The solid neutralized the reaction solution, extracted with ethyl acetate to collect the organic phase, and washed with anhydrous Na 2 SO 4 After drying and filtering, the obtained solid crude product was recrystallized with 15 mL of methanol solution, and suction filtered to obtain 3.424 g of bright red solid powder, namely compound 1, with a yield of 78%. Melting point: 256 – 258 °C; HRMS (LC / MS) m / z: [M + H] + = 438.1705; 1 H NMR (400 MHz, DMSO-d 6 , ppm) δ 9.93 (S, 1H), 8.54...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com