Application of betulinic acid derivatives in preparation of drugs inhibiting T cell differentiation
A technology of betulinic acid and cell differentiation, applied in the application field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: drug screening
[0037] The full name of MTT is 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenyltetrazolium romide, and the Chinese chemical name is 3-(4,5-dimethylthiazol-2)-2,5- Diphenyltetrazolium bromide, trade name thiazolium blue, is a yellow dye. MTT colorimetry is a method for detecting cell survival and growth. Its detection principle is that succinate dehydrogenase in the mitochondria of living cells can reduce exogenous MTT to water-insoluble blue-purple crystal formazan (Formazan) and deposit it. in cells.
[0038] method:
[0039] (1) will 2.5×10 5 100 μl of spleen lymphocytes were placed in a 96-well plate, and three replicate wells were set up for each group.
[0040] (2) Add MOG in vitro 35-55 The final concentration of stimulation (blank control) was 20 μg / ml, and drugs of different concentrations were added.
[0041] (3) The cells were cultured in a 37°C, 5% CO2 incubator for 72 hours.
[0042] (4) Add 20 μl MTS to each well, and pla...
Embodiment 2
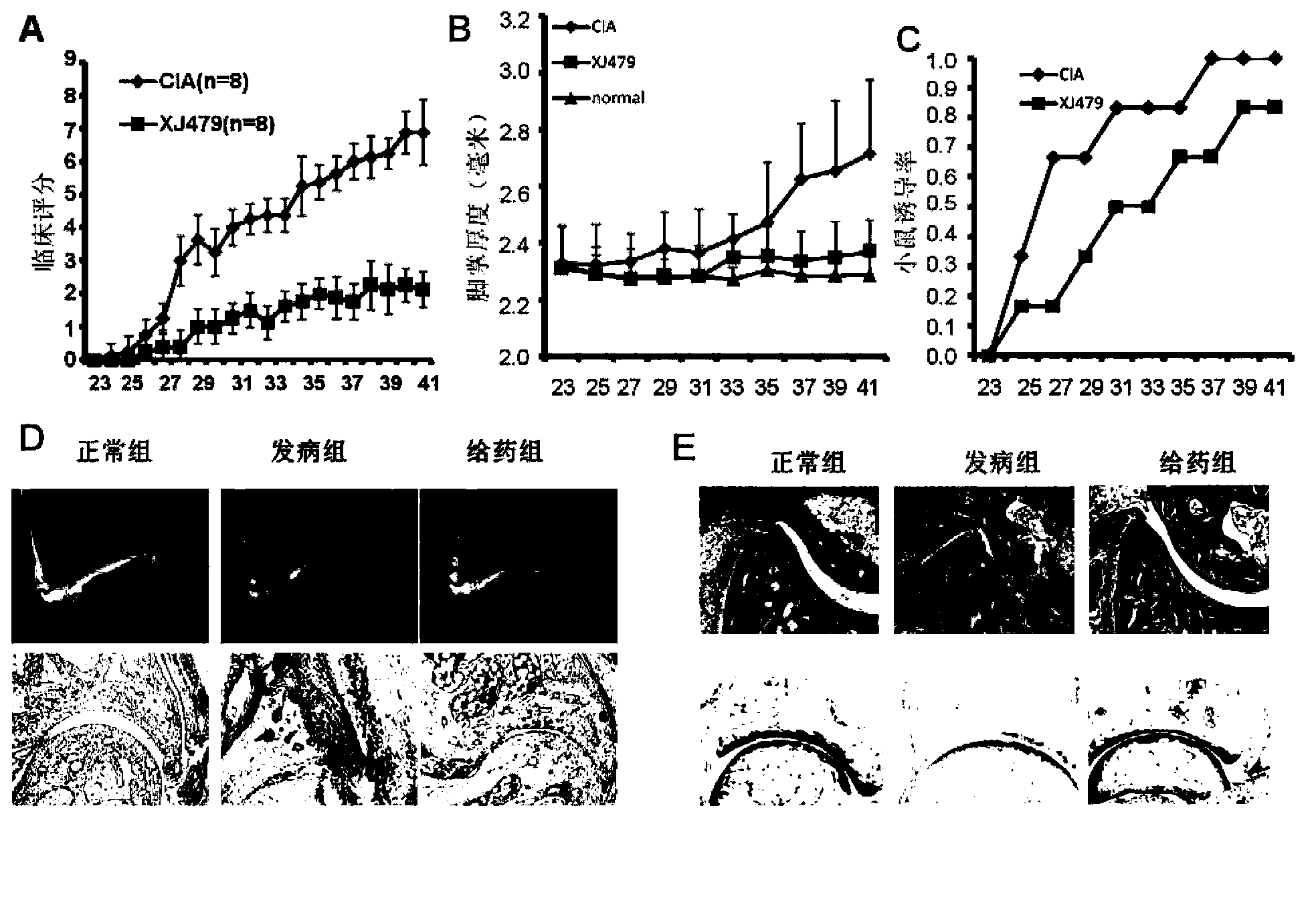
[0047] Example 2: Administration of XJ479 in CIA can alleviate the severity of its onset
[0048] Principle: Administration of betulinic acid derivative XJ479 represented by formula (1) in vivo to alleviate the onset of CIA (inflammatory response, cartilage and bone damage)
[0049] Methods: Male DBA / 1J mice (6-8 weeks) were injected with fully emulsified CII to induce CIA model. Three groups were set up, with 8 mice in each group, and all mice except the normal group were subcutaneously injected with antigen CII at a dose of 100 μg / mouse. The day of immunization was day 0, and the second immunization was performed after day 21. XJ479 (20 mg / kg / day, dissolved in 50 μl DMSO) was administered intraperitoneally on day 23, and the corresponding control group was injected with an equivalent amount of 50 μl DMSO. And the mice were observed from the 22nd day, and the severity of the disease and the degree of joint swelling of the mice were recorded. After the mice reached the peak ...
Embodiment 3
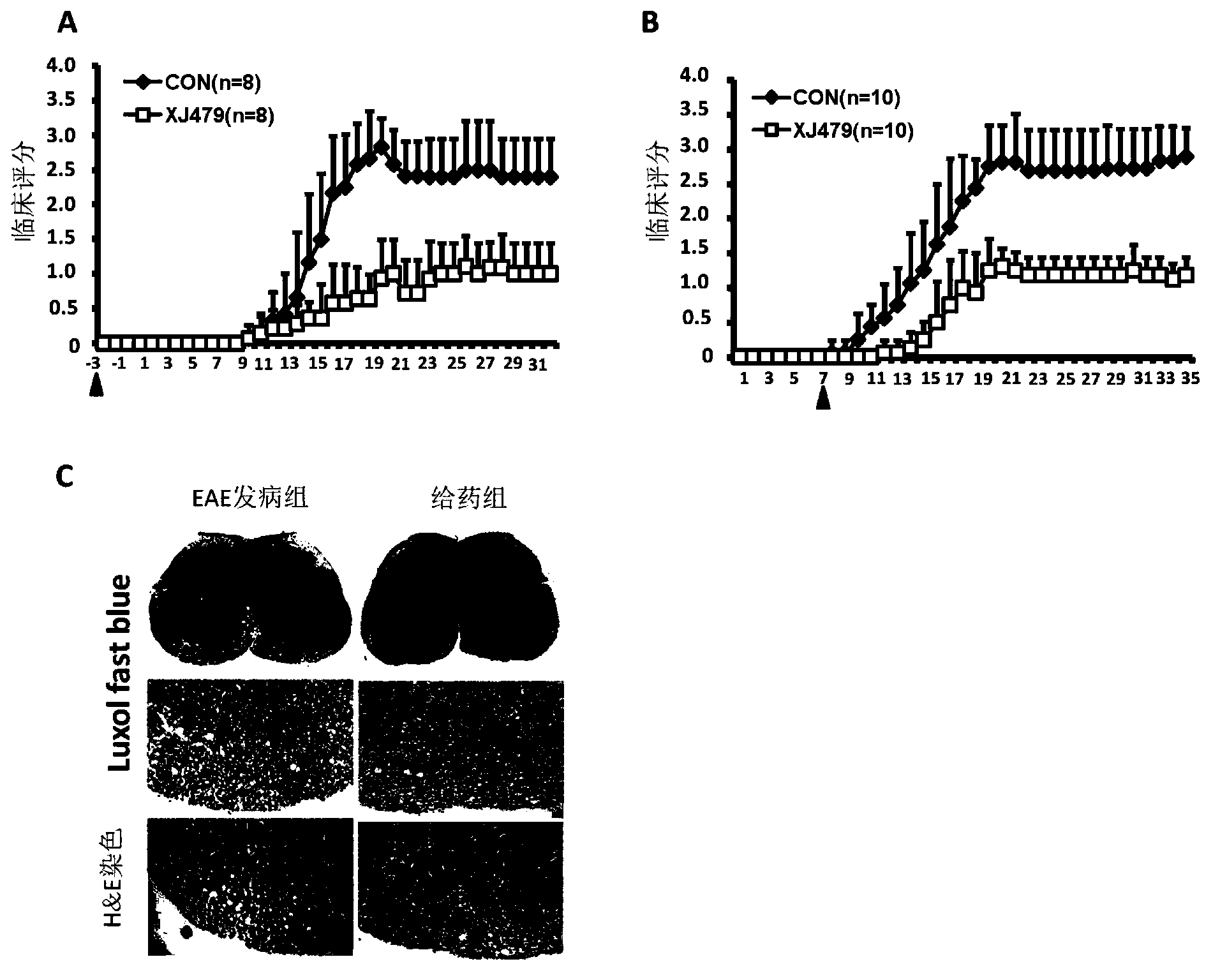
[0051] Example 3: Administration of XJ479 in EAE can alleviate the severity of its onset
[0052] Principle: Administration of betulinic acid derivative XJ479 represented by formula (1) in vivo to alleviate the onset of EAE (infiltration of inflammatory cells in the central nervous system and relief of inflammatory response)
[0053]Method: Prepare 1) complete Freund's adjuvant, add heat-inactivated Mycobacterium tuberculosis (H37RA) to the incomplete Freund's adjuvant to make the final concentration 5 mg / ml, and mix well. 2) MOG polypeptide: Dissolve MOG with 1×PBS (PH7.2) to make a final concentration of 3 mg / ml. 3) Pertussis toxin (PTX): Dissolve PTX with 1×PBS (PH7.2)+50mM NaCl to prepare the final concentration. Then emulsify the antigen, put complete Freund's adjuvant into an emulsification tube, and put a three-way valve on it. Put an equal volume of dissolved MOG into another emulsification tube, connect it with complete Freund's adjuvant with a three-way valve after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com