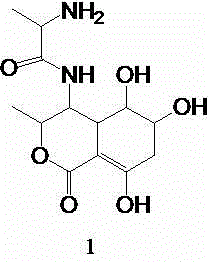
Synthesis method of isocoumarin derivative
A technology of coumarin and compounds, applied in the field of preparation and separation and purification of isocoumarin derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] With cuprous iodide as catalyst and DMF as solvent
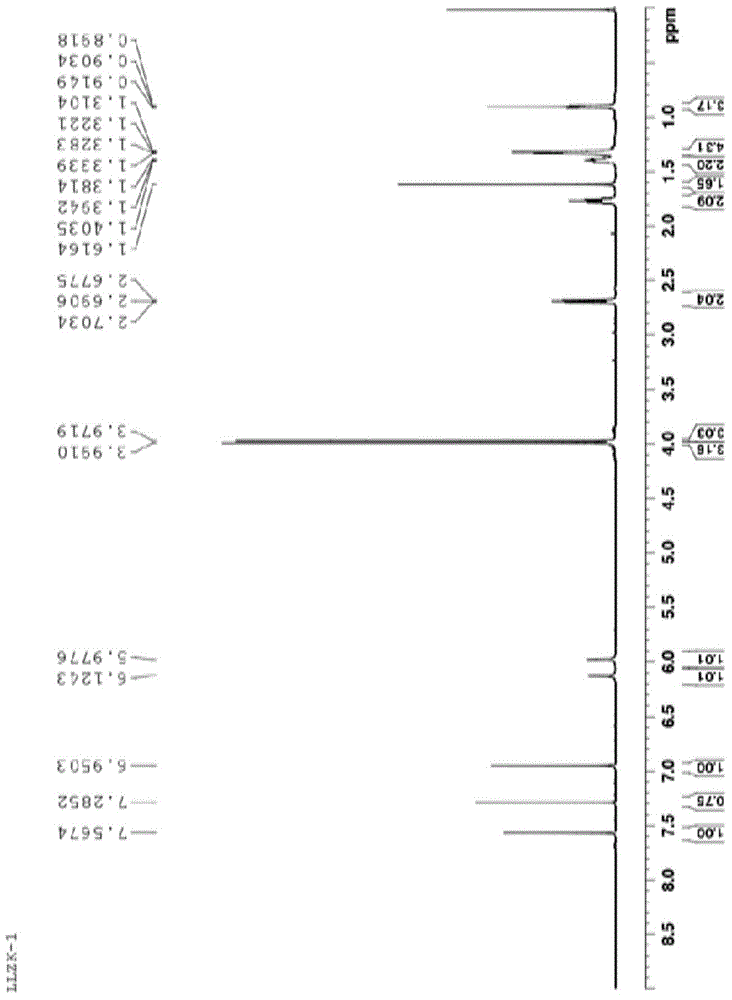
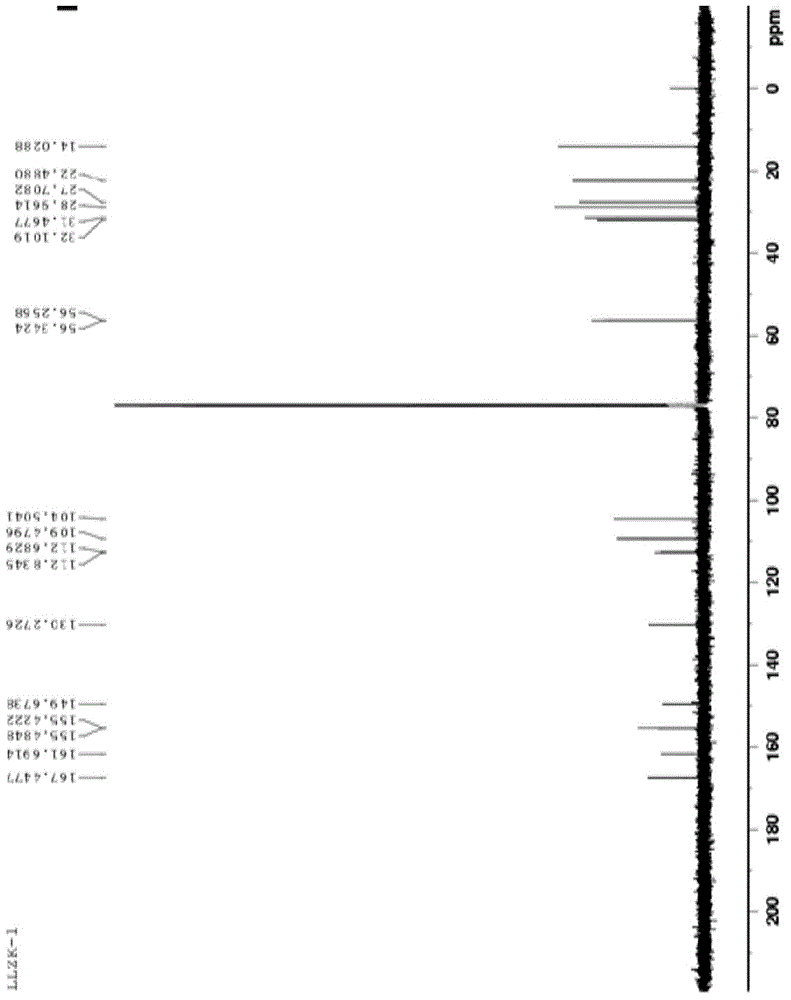
[0023] Add 3.94g of 3-hexyl-4-cyano-6,7-dimethoxyisocoumarin, 1g of cuprous iodide, 15ml of DMF, and 3g of acetaldehyde oxime in sequence into a 100ml reaction vessel, and heat to reflux for 0.5h with an electric heating mantle . After the reaction system is cooled, filter with suction, add 30ml of water to the filtrate in the lower layer, a large amount of solids are precipitated, filter with suction, dissolve the filtered solid with 20ml of ethyl acetate, heat to boil, discard the black solid in the upper layer with suction, and spin evaporate the filtrate in the lower layer to dryness Then add 8ml of ethyl acetate and freeze overnight at -15°C in a freezer. After suction filtration, 2.4 g of off-white solid was obtained. The melting point is 159-162.1°C. The result of NMR characterization is: 1H NMR (600 MHz, CDCl3): δ 7.57 (s, 1H, ArH),7.29(s,2H,NH2)6.95 (s, 1H, ArH),3.99 [s, 3H, ( OCH3)], 3.97 [s, 3H, (OCH3...
Embodiment 2
[0025] With cuprous iodide as catalyst DMF and ethyl acetate as solvent
[0026] Add 3.94g of 3-hexyl-4-cyano-6,7-dimethoxyisocoumarin, 1g of cuprous iodide, 10ml of DMF + 5ml of ethyl acetate, 3g of acetaldehyde oxime into a 100ml reaction vessel, and use a heating mantle to Heat to reflux for 1h. After the reaction system is cooled, filter with suction, add 30ml of water to the filtrate in the lower layer, a large amount of solids are precipitated, filter with suction, heat and dissolve the filtered solid with 20ml of ethyl acetate, discard the solid in the upper layer by suction filtration while it is hot, and spin evaporate the filtrate in the lower layer to dryness Add 8ml of ethyl acetate and freeze overnight at -15°C in a freezer. After suction filtration, 2.75 g of off-white solid was obtained. The melting point is 160-162.3° C., and the retention time detected by high performance liquid chromatography is consistent with Example 1.
Embodiment 3
[0028] Cuprous bromide as catalyst
[0029] Add 3.94 g of 3-hexyl-4-cyano-6,7-dimethoxyisocoumarin, 1 g of cuprous bromide, 15 ml of DMF, and 3 g of acetaldehyde oxime in sequence into a 100 ml reaction vessel, and heat to reflux for 45 min with a heating mantle. After cooling the reaction system, filter with suction, add 30ml of water to the filtrate in the lower layer, a large amount of solids precipitate, filter with suction, dissolve the filtered solid with 8ml of ethyl acetate, and freeze overnight at -15°C in a freezer. After suction filtration, 3.19 g of off-white solid was obtained. The melting point is 161-163.3° C., and the high-performance liquid chromatography retention time is consistent with that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com