Method for adsorbing heavy metal ions in environment by utilizing macroalgae
A technology of heavy metal ions and seaweed, applied in chemical instruments and methods, adsorption water/sewage treatment, seawater treatment, etc., can solve the problems of slow desedimentation rate of heavy metal ions, complicated operation, secondary pollution, etc., and achieve low cost and high distribution Broad, long-growing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
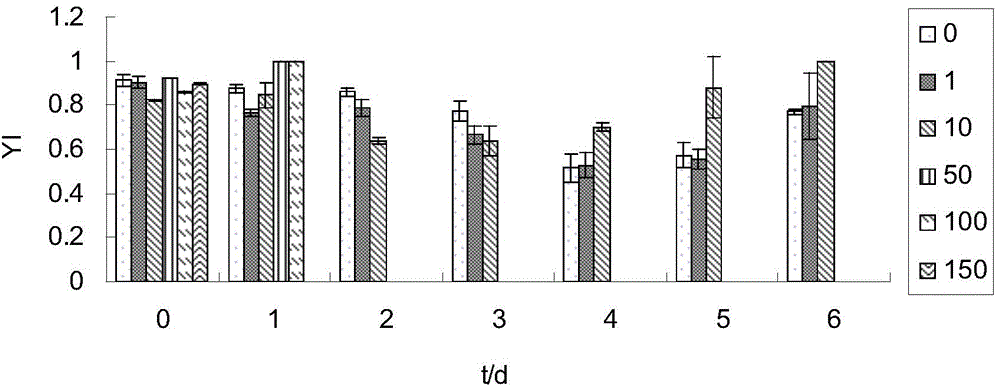
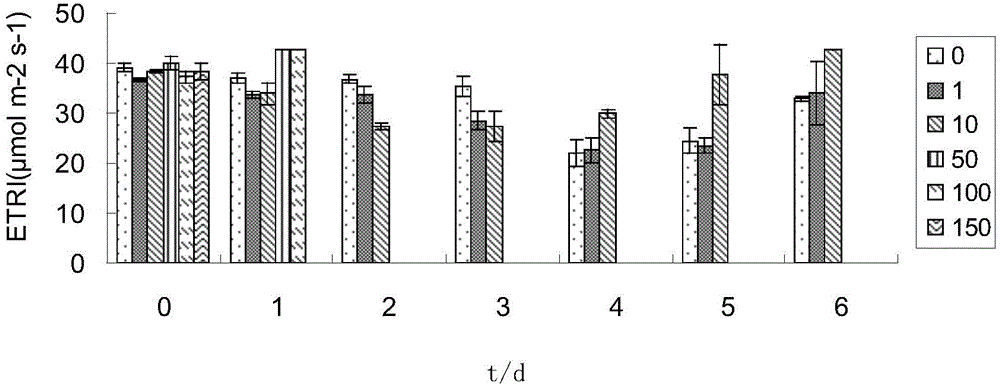
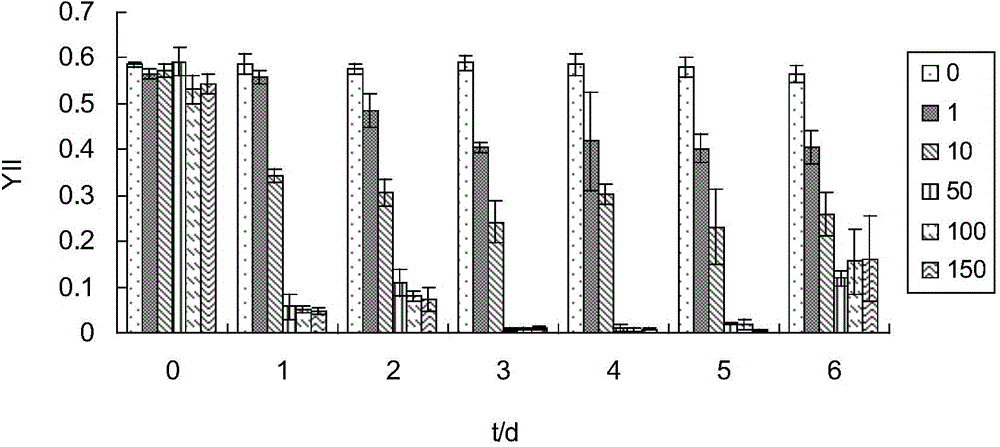
[0037] (1) Prepare 200ml of freshwater culture solution with cadmium ion concentration of 0, 10, 50, 100, and 150μmol / L (fresh water is to use tap water to expose to the sun for 3 hours to remove chloride ions), and then place them in 250ml Erlenmeyer flasks ,stand-by.
[0038] (2) Add 4g of fresh Enteromorpha per bottle.
[0039] (3) After each sample was treated at room temperature for 1d, 2d, and 3d, the photosynthetic activity (Dual-PAM) of the algae was measured (the photosynthetic activity was measured at room temperature, and the results are shown in Figure 1-5 ), and respectively take 2ml of the culture solution after the above treatment, and measure the content of cadmium ions (results in Figure 6 ).
[0040] (4) Calculate the adsorption rate, calculate the adsorption rate method, the adsorption rate (%)=100*(C 0 -C e ), (C 0 is the original cadmium ion content, C e is the remaining cadmium ion content).
[0041] by the above Figure 1-5 It can be seen that ...
Embodiment 2
[0049] (1) Prepare 200ml of semi-seawater (half-seawater refers to a certain amount of seawater mixed with an equal amount of distilled water) culture solution with cadmium ion concentrations of 0, 10, 50, 100, and 150 μmol / L, and then place them in 250ml of In the triangular flask, set aside.
[0050] (2) Add 4g of fresh Enteromorpha per bottle.
[0051] (3) Each sample was treated at room temperature for 1d, 2d, 3d, 4d, 5d, 6d, 7d, 8d, and 9d to measure the photosynthetic activity (Dual-PAM) of the algae (the photosynthetic activity was measured at room temperature, and the results were See Figure 7-11 ), and respectively take 2ml of the culture solution after the above treatment, and measure the remaining cadmium ion content (results see Figure 12 ).
[0052] (4) Calculate the adsorption rate, adsorption rate (%)=100*(C 0 -C e ), (C 0 is the original cadmium ion content, C e is the remaining cadmium ion content)
[0053] by the above Figure 6-11 It can be seen t...
Embodiment 3
[0061] (1) Prepare 200ml of seawater culture solution with cadmium ion concentration of 0, 10, 50, 100, and 150μmol / L respectively, and then place them in (250ml) Erlenmeyer flasks for use.
[0062] (2) Add 4g of fresh Enteromorpha per bottle.
[0063] (3) Each sample was treated at room temperature for 1d, 2d, 3d, 4d, and 5d to measure the photosynthetic activity of the algae (Dual-PAM). The photosynthetic activity was measured at room temperature, and the results are shown in Figure 13-17 , and take 2ml of the above-mentioned treated culture solution respectively, and measure the remaining cadmium ion content (results see Figure 18 ).
[0064] (4) Calculate the adsorption rate, adsorption rate (%)=100*(C 0 -C e ), C 0 is the original cadmium ion content, C e is the remaining cadmium ion content)
[0065] by the above Figure 13-17 It can be seen that 0d is the time for processing materials, 1-5d is the process of processing, 6-8d is the recovery process, and the rec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com