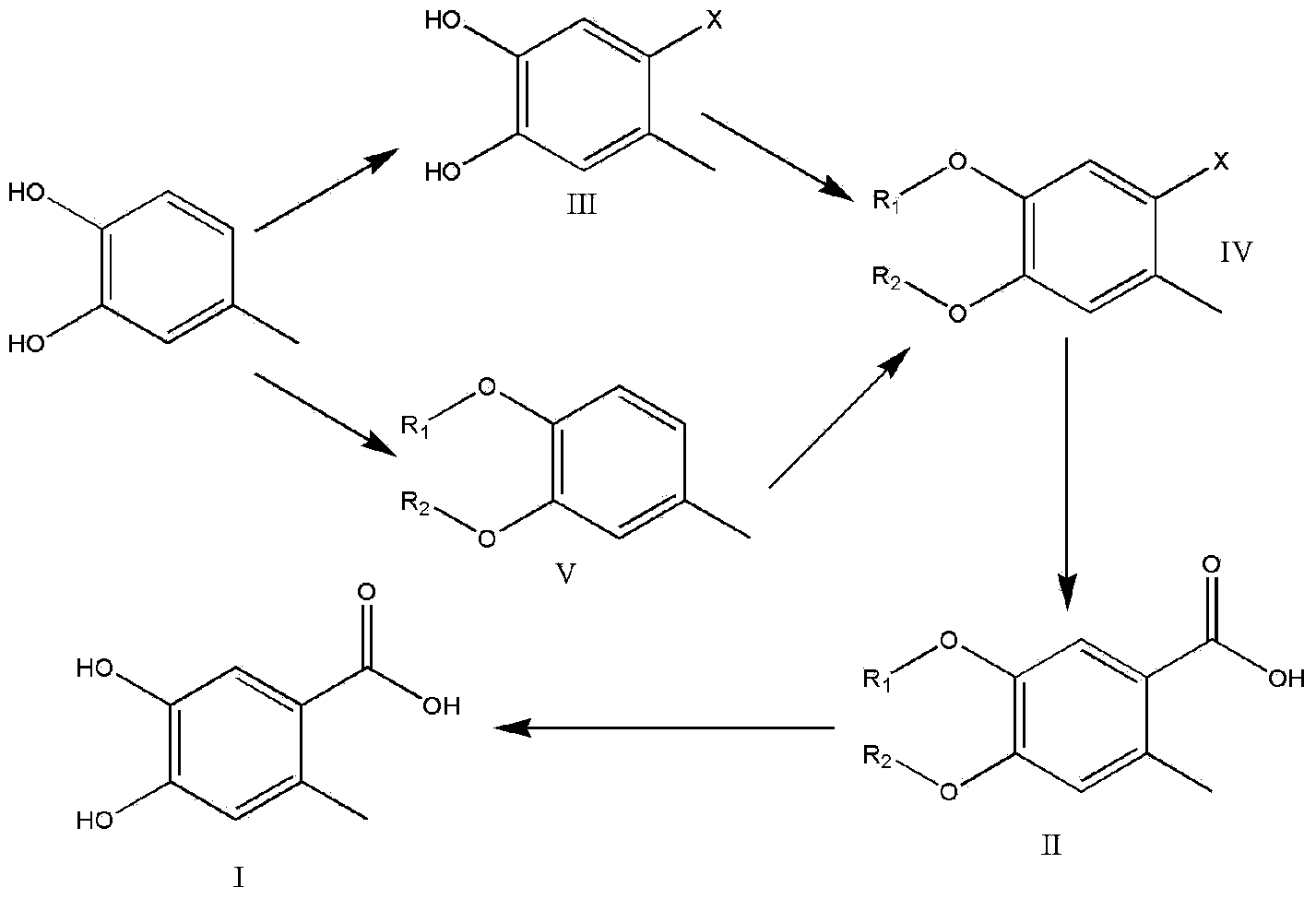
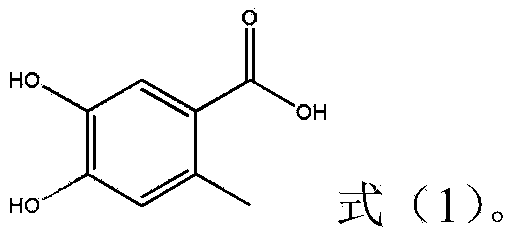
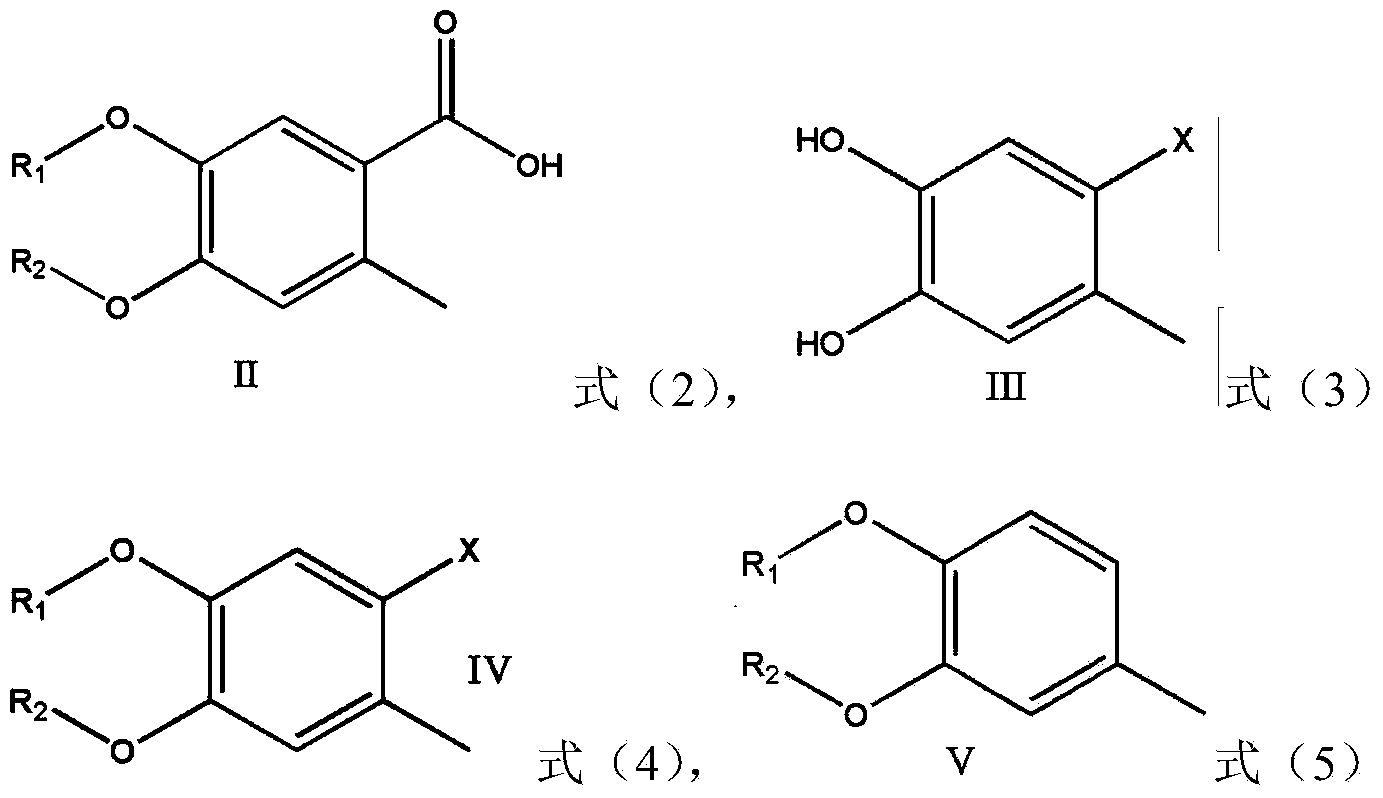
Preparation method of 4,5-dihydroxyl-2-methyl benzoic acid
A toluic acid and dihydroxyl technology, applied in the fields of fine chemicals and pharmaceutical intermediates, can solve the problems of heavy pollution, complicated steps, difficult to obtain raw materials, etc., and achieve the effects of simple and safe operation, easy access to raw materials, and easy products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 prepares intermediate III
[0052] Take the preparation of 2-bromo-4,5-dihydroxytoluene as an example
[0053] Into a 500 ml three-necked round bottom flask equipped with a mechanical stirrer, 24.8 g of 4-methylbenzene-1,2-diol (0.2 mol) and 200 ml of dichloromethane were added and stirred to form a solution. Place the reaction flask in a cooling tank of acetone and dry ice and keep stirring. 38.4g of bromine (0.24mol) was added dropwise within half an hour, and the temperature in the reaction flask was kept at -70°C to -30°C during the dropwise addition. After the dropwise addition, the cooling tank was removed and the temperature was naturally raised to room temperature. The solvent was evaporated to dryness, recrystallized, filtered, and sucked dry to obtain 38.2 g of product with a yield of 94.2% and a purity of 93.8%. The crude product was recrystallized from ethyl acetate / petroleum ether (1:4) to obtain 35.8 g of a solid with a purity of 98.1%.
[...
Embodiment 2
[0055] Embodiment 2 prepares intermediate V
[0056] Take the preparation of 3,4-dibenzyloxytoluene as an example
[0057] To a 500 ml three-neck round bottom flask equipped with a mechanical stirrer, first add 66.7 g of potassium carbonate (0.48 mol), 24.8 g of 4-methylbenzene-1,2-diol (0.2 mol) and 250 ml of acetone, and stir for half Hour. 60.8 g of benzyl chloride (0.48 mol) was added to the mixture, heated to reflux for 5 hours, and the reaction was tracked by TLC. After the reaction, filter, add water to stir, filter under reduced pressure, collect the solid, and drain to obtain 56.9 g of a light yellow solid with a yield of 93.4% and a purity of 95.4%.
[0058] If the same mole of benzyl bromide is used instead of benzyl chloride, 57.4 g of solids are obtained with a purity of 96.1%.
Embodiment 3
[0059] Embodiment 3 prepares intermediate IV
[0060] Taking intermediate V as raw material to prepare 4,5-dibenzyloxy-2-bromotoluene as an example
[0061] Into a 500 ml three-neck round bottom flask equipped with a mechanical stirrer, add 30.4 g of intermediate V (0.1 mol) and 200 ml of dichloromethane, and stir to form a solution. Place the reaction flask in a cooling tank of acetone and dry ice and keep stirring. Within half an hour, 19.2 g of bromine (0.12 mol) was added dropwise to the solution. During the dropwise addition, the temperature in the reaction flask was kept at -70°C to -30°C. After the dropwise addition, the cooling tank was removed and the temperature was naturally raised to room temperature. The solid was collected by filtration under reduced pressure and sucked dry to obtain 36.5 g of a light yellow solid with a purity of 96.4% and a yield of 95.3%.
[0062] If NaBr-Br with bromine equimolar 2 Using the same operation method instead of bromine, 37.1 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com