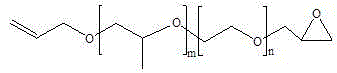
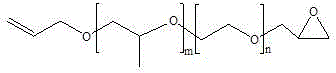Preparation method of glycidol ether base end-capped allyl alcohol random polyether
A glycidyl ether-based, random polyether technology, applied in the chemical industry, can solve the problems of troublesome solvents, poor product color, low conversion rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
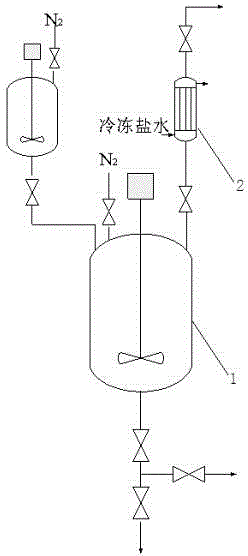
[0052]Put 56.47g (2.353moL) of NaH and 239.46g (2.588moL) of epichlorohydrin into the dripping reaction kettle with nitrogen, stir for 30min and fill with nitrogen to 0.4 MPa; Add 1000g (2.353moL) allyl alcohol atactic polyether (molecular weight=425, m=2.62, n=4.63), 0.782g (0.002353moL) tetra-n-butylammonium bromide, set nitrogen under vacuum, and open the nitrogen valve and the outlet valve of the reflux condensing device, continuously feed nitrogen gas to the end-capping kettle (nitrogen flows through the capping kettle to the reflux condensing device, and finally enters the tail gas device through the outlet valve); open the connecting valve, and press the dropping addition kettle into the end-capping kettle. About 1h; after the dropwise addition, the temperature was raised to 40°C, and the reaction was carried out at this temperature for 5h. After the reaction is completed, the temperature is lowered to 30°C, and the crude product is obtained so far; the crude product is...
Embodiment 2
[0055] Put 29.27g (1.22moL) of NaH and 117.34g (1.27moL) of epichlorohydrin into the dripping reactor with nitrogen, stir for 30min and then fill with nitrogen to 0.4 MPa; Add 1000g (0.976moL) allyl alcohol random polyether (molecular weight = 1025, m = 15.4, n = 5.0), 0.808g (0.00488moL) tetraethylammonium chloride, after vacuum nitrogen, open the nitrogen valve and The outlet valve of the reflux condensing device continuously feeds nitrogen gas to the end-capping kettle (nitrogen flows through the capping kettle to the reflux condensing device, and finally enters the tail gas device through the outlet valve); open the connecting valve, and the dripping kettle presses the material into the capping kettle for about 1h; after the dropwise addition, the temperature was raised to 60°C, and the reaction was carried out at this temperature for 8h. After the reaction was completed, the temperature was lowered to 30°C, and the crude product was obtained; after the crude product was f...
Embodiment 3
[0057] Put 17.78g (0.74moL) of NaH and 68.53g (0.74moL) of epichlorohydrin into the dripping reaction kettle with nitrogen, stir for 30min and fill with nitrogen to 0.4 MPa; Add 1000g (0.494moL) allyl alcohol random polyether (molecular weight = 2025, m = 22.35, n = 16.96), 1.372g (0.00494moL) tetrabutylammonium chloride, after vacuum nitrogen, open the nitrogen valve and The outlet valve of the reflux condensing device continuously feeds nitrogen gas to the end-capping kettle (nitrogen flows through the capping kettle to the reflux condensing device, and finally enters the tail gas device through the outlet valve); open the connecting valve, and the dripping kettle presses the material into the capping kettle for about 1h; after the dropwise addition, the temperature was raised to 80°C, and the reaction was carried out at this temperature for 10h. After the reaction is completed, the temperature is lowered to 30°C, and the crude product is obtained so far; the crude product i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| color | aaaaa | aaaaa |
| color | aaaaa | aaaaa |
| color | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com