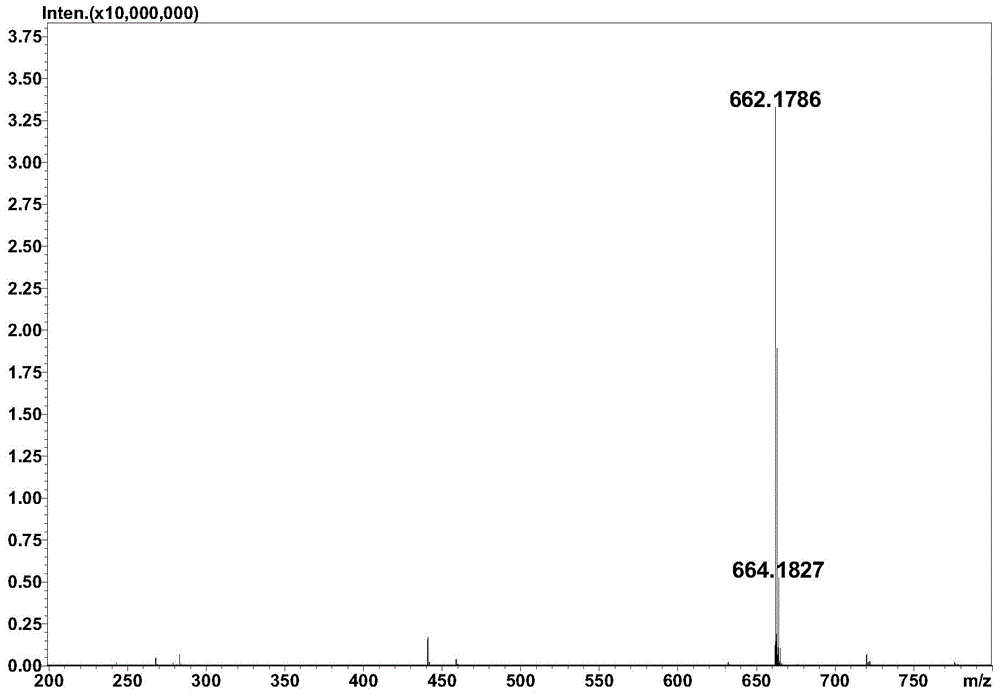
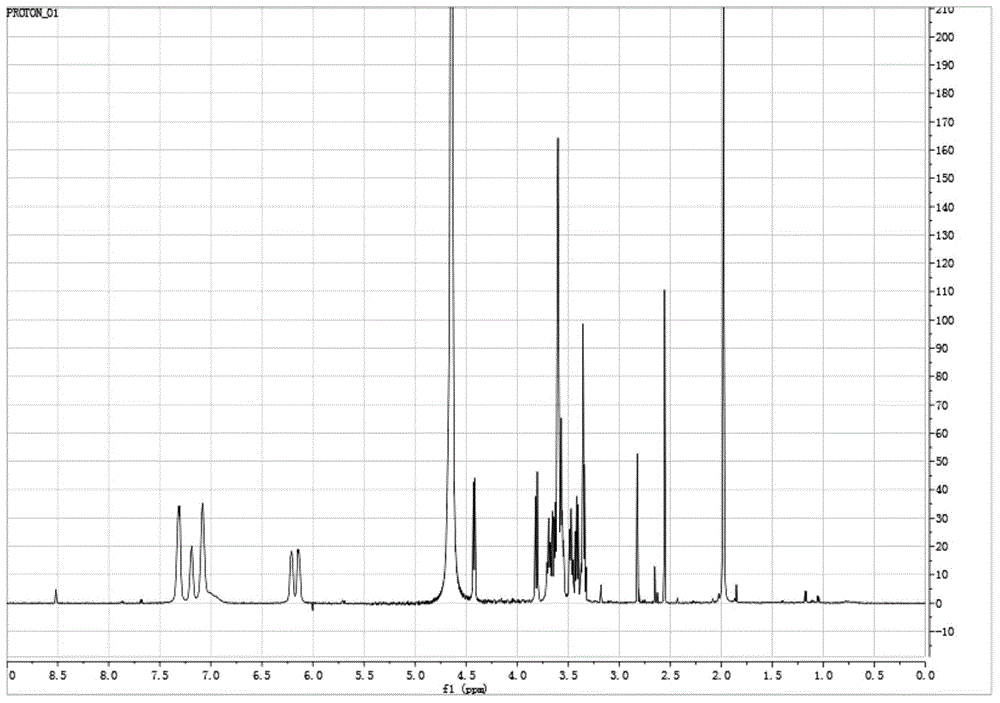
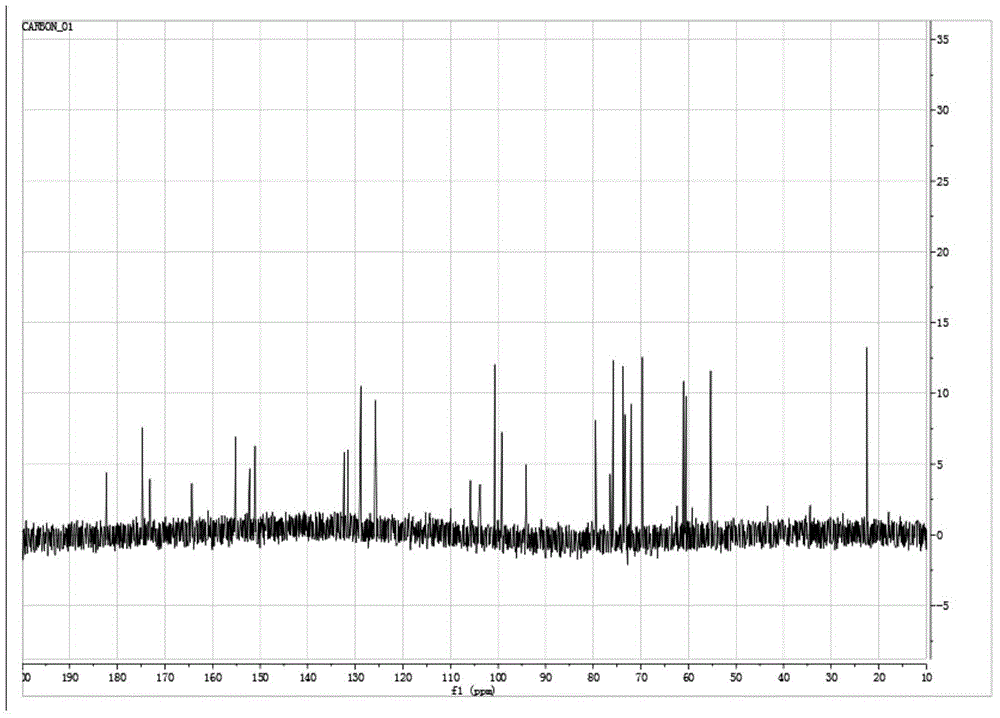
Method for preparing hyaluronan oligosaccharide chain modified flavonoid compound carrying glucuronic acid on terminal
A technology of hyaluronic acid oligosaccharides and glucuronic acid, which is applied in the direction of fermentation, etc., to achieve the effect of simple process, easy operation and stable activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 A preparation method of hyaluronic acid oligosaccharide chain modified flavonoid glycoside compound
[0062] A method for modifying flavonoids with glucuronic acid at the end of a hyaluronic acid oligosaccharide chain, the steps are as follows:
[0063] 1. Preparation of Hyaluronan Synthase
[0064] According to the GenBank accession number AF036004, the hyaluronan synthase gene was obtained by gene synthesis. The target gene was obtained by PCR and constructed into the pET-15b vector, and the recombinant vector obtained by the construction was transformed into the expression strain Escherichiacoli BL21 (DE3), and isopropylthiogalactoside (IPTG) induced the expression of hyaluronic acid synthase; The hyaluronic acid synthase is obtained by ultrasonically disrupting the bacteria, purifying by Ni-NTA affinity chromatography, and concentrating by ultrafiltration. The molecular weight and purity of the target protein were tested by sodium dodecyl sulfate polyacr...
Embodiment 2
[0082] Example 2 A preparation method of a flavonoid compound whose hyaluronic acid oligosaccharide chain modification end is glucuronic acid
[0083] A kind of hyaluronic acid oligosaccharide chain modified end is the preparation method of the flavonoid compound of glucuronic acid as described in embodiment 1, difference is:
[0084] The final molar concentration ratio of the reaction solution of UDP-GlcNAc, UDP-GlcA and hyaluronic acid trisaccharide-modified flavonoids is 100:100:1.
[0085] The product electrophoresis detection results were as follows: Figure 7 Strip 1 is shown.
Embodiment 3
[0086] Example 3 A preparation method of flavonoids whose hyaluronic acid oligosaccharide chains are modified with glucuronic acid at the end
[0087] A kind of hyaluronic acid oligosaccharide chain modified end is the preparation method of the flavonoid compound of glucuronic acid as described in embodiment 1, difference is:
[0088] The final molar concentration ratio of the reaction solution of UDP-GlcNAc, UDP-GlcA and hyaluronic acid trisaccharide-modified flavonoids is 1000:1000:1.
[0089] The product electrophoresis detection results were as follows: Figure 7 Shown in strip 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com