Hydrogen production method
A technology of calcium hydroxide and mayenite, applied in the production of hydrogen, chemical instruments and methods, hydrogen, etc., can solve the problem of large hydrogen volume and achieve high safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The method for preparing hydrogen of the present invention is characterized in that by using mayenite (Mayenite: Ca 12 al 14 o 33 ) and calcium hydroxide [Ca(OH) 2 ] into water so that they react with water to produce hydrogen.
[0025] As mentioned above, putting mayenite and calcium hydroxide into water and reacting them with water produces Katoite [Ca 3 al 2 (OH) 12 ] and hydrogen (H 2 ).
[0026] Ca 12 al 14 o 33-x +9Ca(OH) 2 +(33+x)H 2 O→7Ca 3 al 2 (OH) 12 +xH 2
[0027] In the hydrogen production method of the present invention, the temperature of the water is 50-100° C., and the molar ratio of mayenite to calcium hydroxide is preferably 1:9.
[0028] Here, when the temperature of water in the method for producing hydrogen is lower than 50° C., the hydrogen production reaction rate becomes slow and the yield deteriorates, which is not preferable. In addition, the temperature of water does not exceed 100° C. during the hydrogen generation reaction...
Embodiment 1
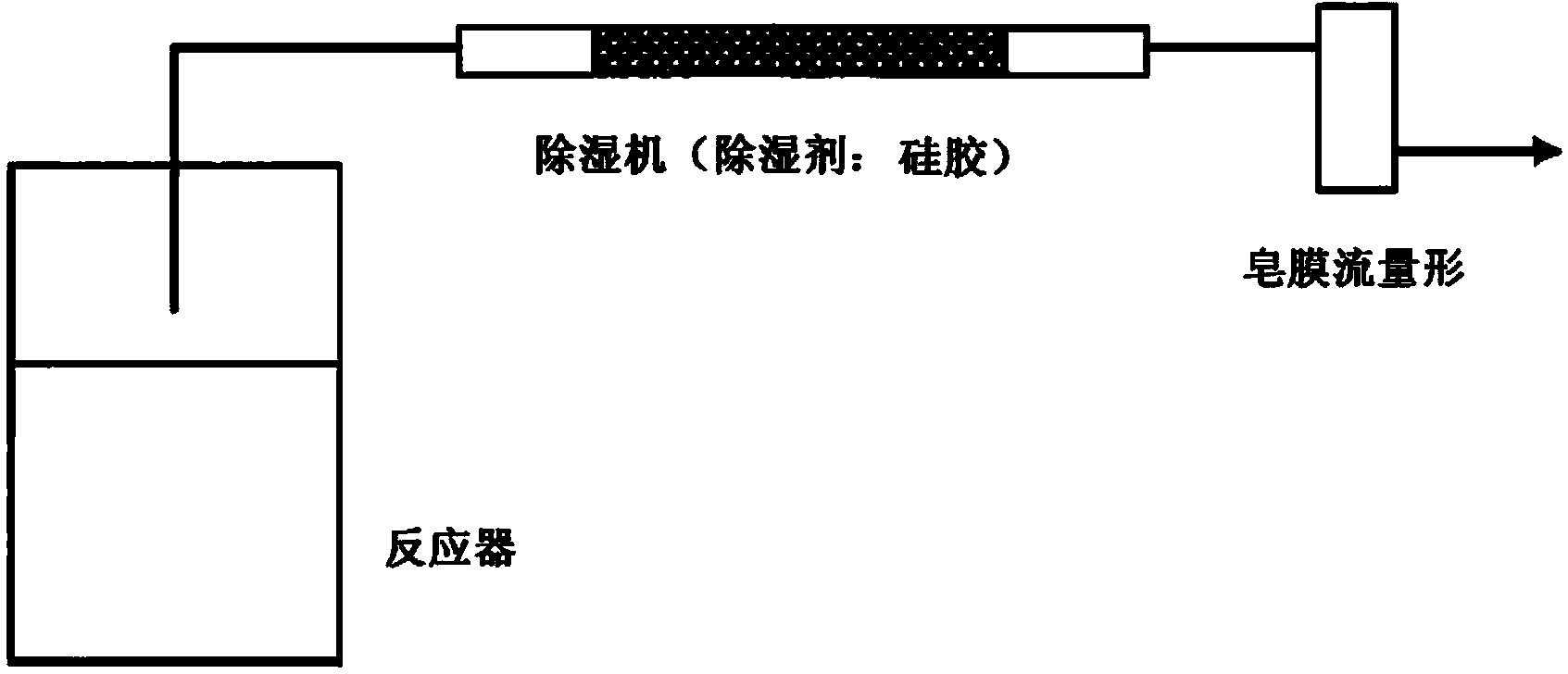
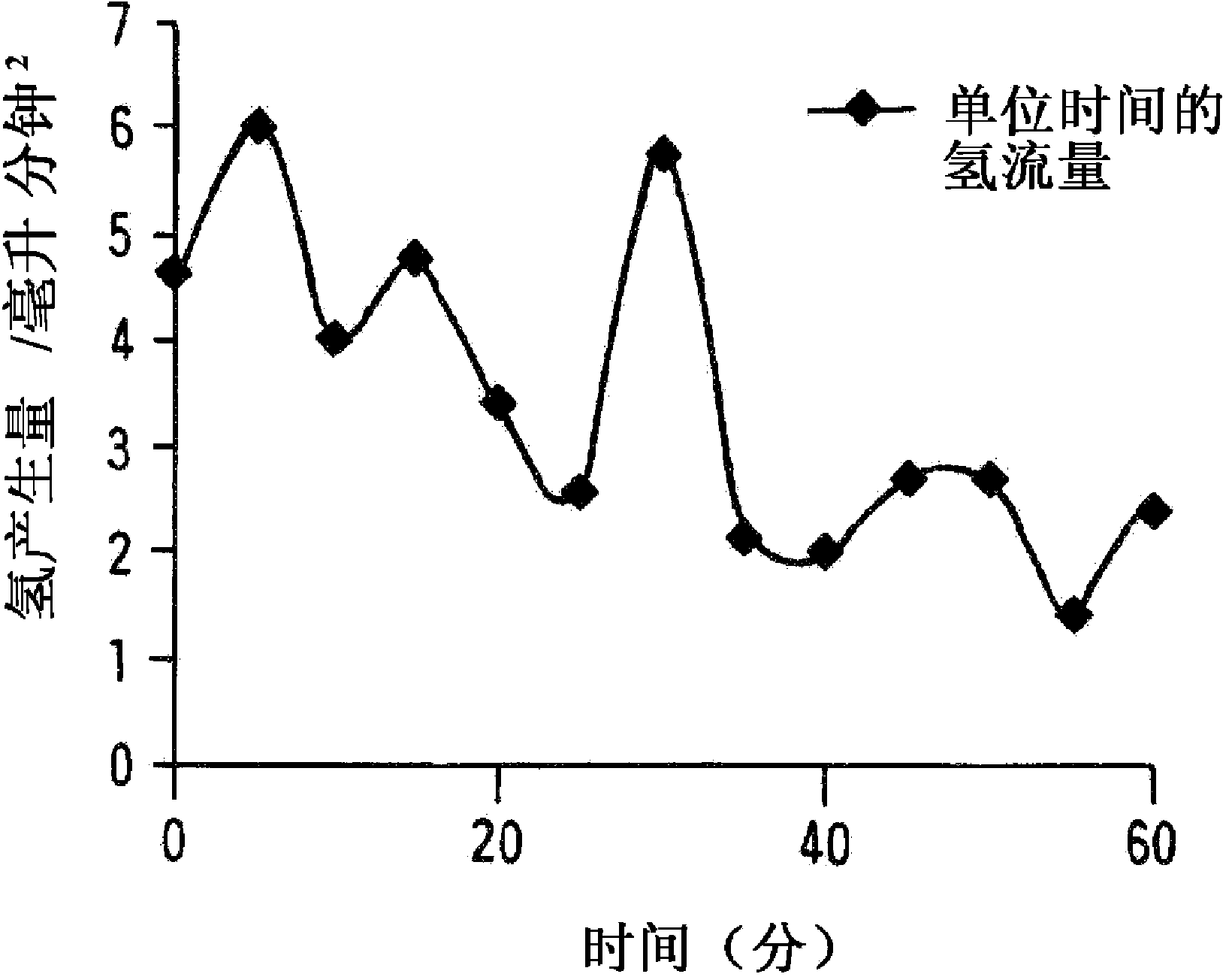
[0034] used figure 1 The test apparatus for hydrogen production shown is for implementing the hydrogen production method of the present invention.
[0035] First, 200 ml of ion-exchanged water was charged into a 1-liter capacity reactor (separable flask). Next, 9g of aluminum powder (trade name #150, manufactured by minalco) and 12g of calcium hydroxide [Ca(OH) 2 ] (manufactured by Wako Pure Chemical Industries, Ltd.) was put into the reactor and stirred. After the generation of hydrogen gas was completed, the ion-exchanged water was filtered, and the filtered solid content was dried in air at a temperature of 70°C.
[0036] The obtained solid component is Katoite, which is roasted in air at a temperature of 300°C for 2 hours to generate mayenite (Mayenite: Ca 12 al 14 o 33 ) and calcium hydroxide.
[0037] 200 ml of ion-exchanged water was charged into a 1-liter capacity reactor (separable flask). Next, the aforementioned mayenite (Ca 12 al 14 o 33 ) and calcium hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com