Liquid crystal compound and liquid crystal mixture containing the compound
A liquid crystal compound and compound technology, applied in the fields of liquid crystal materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of reducing the clearing point of liquid crystal and restricting the space for improving the response speed of liquid crystal mixtures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
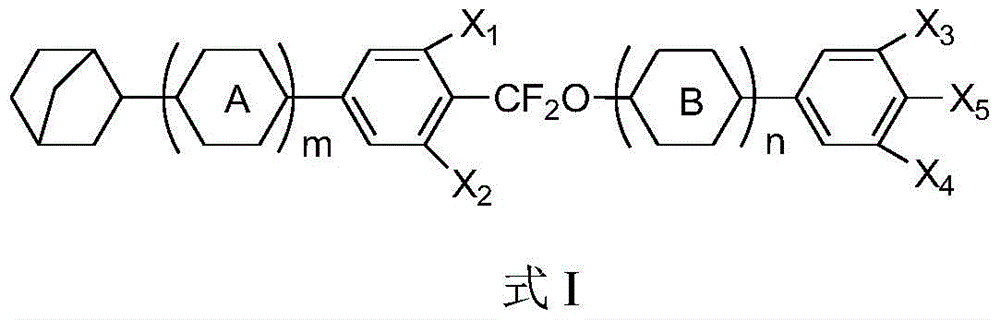
[0027] The preparation method of the compound represented by the above-mentioned formula I is as follows: Synthetic Schemes 1, 2, and 3. Wherein, synthetic route 2 has provided the synthetic method of some intermediates that cannot be obtained in the market, such method principle, operation process, conventional post-treatment, silica gel column, recrystallization purification and other means are well known to those skilled in the art. According to the following introduction, the synthesis process can be completely realized and the target product can be obtained.
[0028] The reaction process generally monitors the progress of the reaction by TLC. The post-treatment after the reaction is generally washed with water, extracted, combined with organic phases, dried, evaporated under reduced pressure to remove solvent, and recrystallization, column chromatography. Those skilled in the art can follow The following description implements the present invention.
[0029] Route 1: i...
Embodiment 1
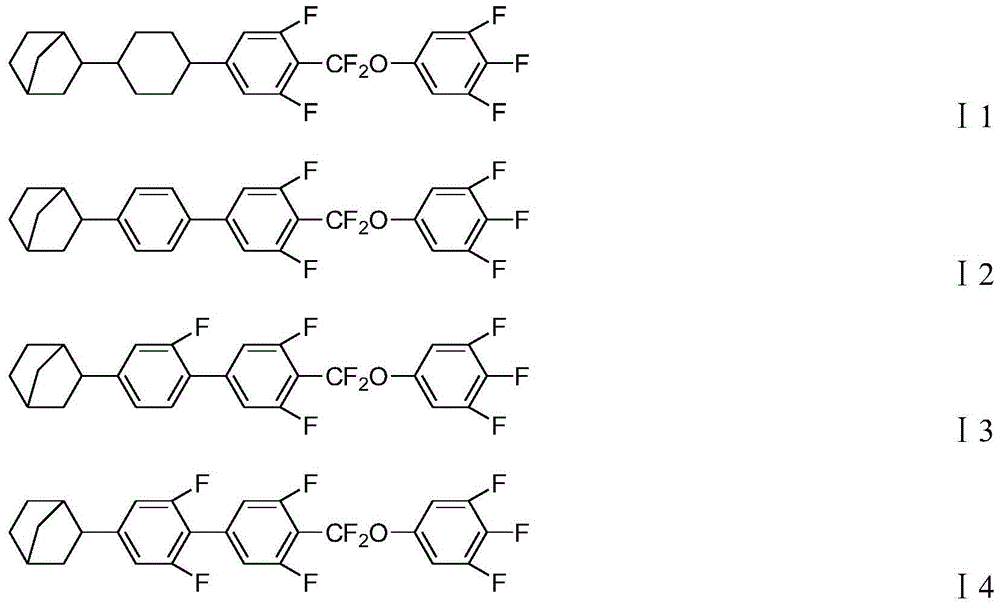
[0065] Embodiment 1, compound shown in preparation formula I2 and formula I6
[0066]
[0067] step 1
[0068]
[0069] 47.2g (0.2mol) of p-dibromobenzene was dissolved in 280ml of tetrahydrofuran, and the temperature was lowered to -70°C under the protection of nitrogen. The reaction solution became more viscous, and 84ml (0.21mol, 2.5M) of n-butyllithium was added dropwise to exchange lithium and bromine. After the addition, stir for another 15 minutes, then add 14 g (0.2 mol) of norcamphor dropwise while maintaining the temperature, gradually become thinner, transparent when the addition is completed, heat up to 0 ° C, pour into 300 ml of water, separate the organic layer, extract, Wash with water and evaporate the solvent. Add 200ml of toluene and 1.8g of p-toluenesulfonic acid, heat up to 110°C and reflux to separate water. Pour it into 200ml of water, separate the organic layer, extract, wash the obtained product with water, evaporate the solvent, and recrystalli...
Embodiment 2
[0093] Embodiment 2, compound shown in preparation formula I1
[0094]
[0095] step 1
[0096]
[0097] Dissolve 67.2g (0.35mol) of 3,5-difluorobromobenzene in 100ml of tetrahydrofuran for use, add 8.4g (0.35mol) of magnesium chips and 100ml of tetrahydrofuran into a 500ml three-necked flask and heat to reflux, then add a small amount of the above solution dropwise to prepare Grignard Reagent, after the reaction is initiated, keep reflux and add dropwise (if it is difficult to initiate the reaction, you can add iodine particles or ethyl bromide to initiate), reflux for one hour after the addition, and after the 3,5-difluorobromophenyl Grignard reagent is obtained, cool down in a water bath Add 53.2g (0.35mol) of 4-bromobicyclo[2.2.1]heptylcyclohexanone dropwise, and reflux for one hour after the addition. The viscous reaction solution was obtained, then poured into 300ml of ice water and 30ml of hydrochloric acid, hydrolyzed under stirring, separated, extracted, washed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com