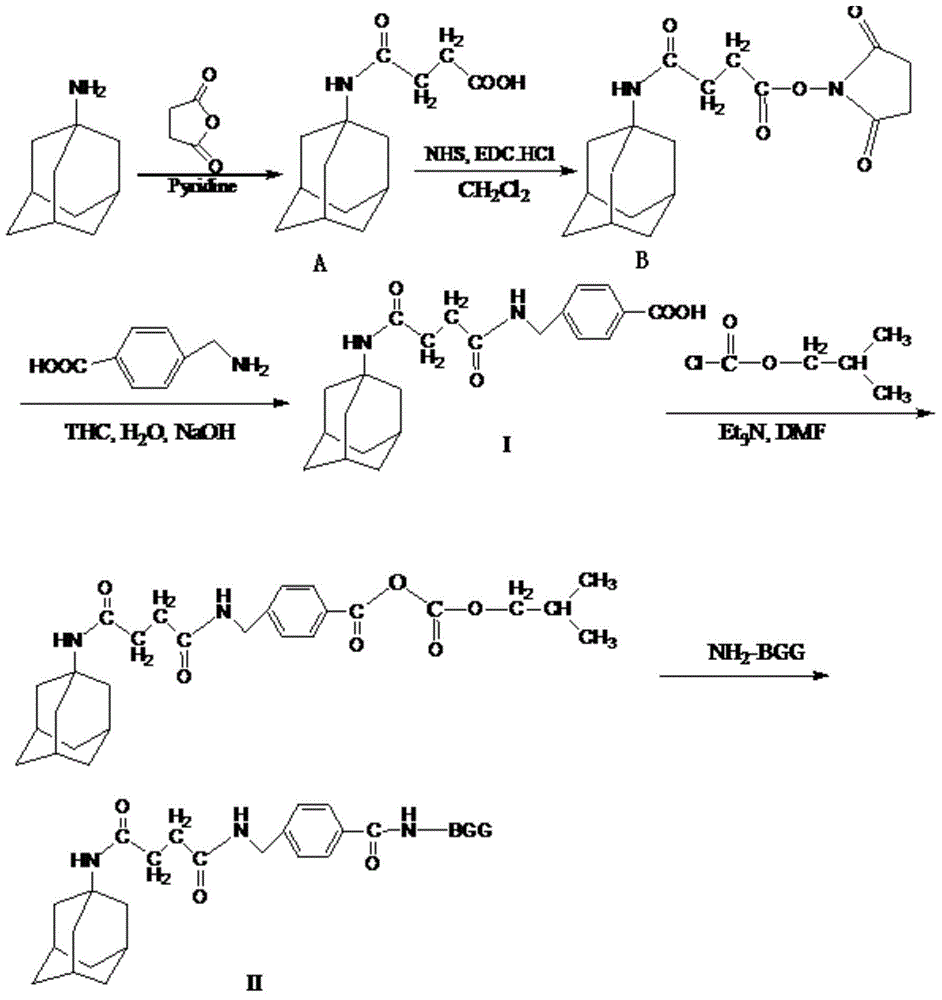
Amantadine artificial hapten and artificial antigen as well as preparation method and application of amantadine artificial hapten and artificial antigen
An artificial hapten, amantadine technology, applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of artificial hapten and artificial antigen without amantadine, and achieve immunogen Good sex, strong specificity, good specificity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This implements a kind of preparation method of amantadine artificial antigen (reaction process is as follows: figure 1 ), including the following steps:
[0056] (1) Preparation of artificial hapten:
[0057] ① Weigh 200mg of amantadine (1.322mmol), 198mg of succinic anhydride (1.980mmol) in a 100ml single-necked round bottom flask, add 10ml of pyridine, add a stirrer, heat the oil bath to 100°C and stir under reflux for 16 hours; After the end, the solvent was evaporated to dryness under reduced pressure, the residue was dissolved with 15ml of double distilled water, the aqueous solution was extracted with 3×15ml of dichloromethane, the organic phases were combined and washed with 15ml of double distilled water, the organic phase was dried over anhydrous magnesium sulfate, filtered, and transferred to After drying, 252 mg of product A was obtained as a colorless oil;
[0058] The colorless oily product A is detected by TLC, the chromatographic solution is an aqueous...
Embodiment 2
[0075] This implements a kind of preparation method of amantadine artificial antigen (reaction process is as follows: figure 1 ), including the following steps:
[0076] (1) Preparation of artificial hapten:
[0077] ① Weigh 200mg of amantadine (1.322mmol) and 145.6mg of succinic anhydride (1.455mmol) into a 100ml single-necked round bottom flask, add 10ml of pyridine, add a stirrer, heat the oil bath to 105°C and stir under reflux for 17 hours; After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, the residue was dissolved with 15ml of double distilled water, the aqueous solution was extracted with 3×15ml of dichloromethane, the organic phases were combined and washed with 15ml of double distilled water, the organic phase was dried over anhydrous magnesium sulfate, filtered, After drying, 251.5 mg of product A was obtained as a colorless oil;
[0078] The colorless oily product A is detected by TLC, and the chromatographic solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com