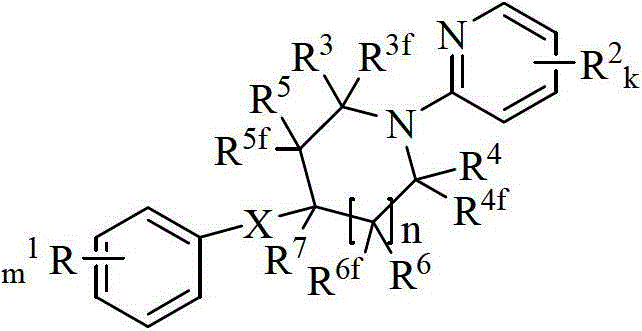
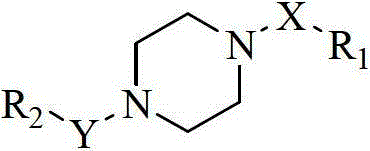
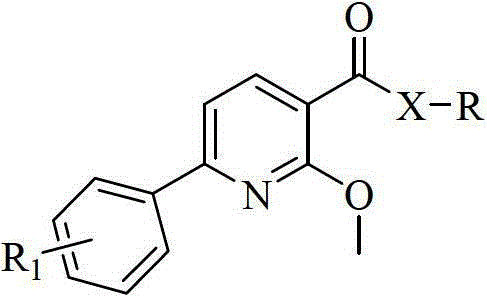
Aryl pyridine/pyrimidine compound and application thereof
A kind of technology of aryl pyridine compounds, applied in the field of aryl pyridine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0556] Example 1: Preparation of 2-methyl-5-cyano-6-(4-chlorophenyl)-nicotinoyl chloride
[0557] 1) Preparation of 2-methyl-5-cyano-6-hydroxy-nicotinic acid ethyl ester
[0558]
[0559] Add 100.0g (0.54mol) ethyl 2-ethoxymethylene acetoacetate and 45.2g (0.54mol) cyanoacetamide into a three-necked flask containing 150mL ethanol, and then add 36.5g (0.54mol) ethanol to it Sodium was reacted at room temperature for 5 hours. After the reaction was monitored by TLC, the solvent was removed under reduced pressure, the residue was poured into water, and the pH was adjusted to 2 with 20% hydrochloric acid. A large amount of solid precipitated out, filtered with suction, the filter cake was washed with water, and air dried to give white Solid 50.8g, yield: 45.9%.
[0560] 2) 2-Methyl-5-cyano-6-chloro-nicotinic acid ethyl ester
[0561]
[0562] Put 50.8g (0.25mol) 2-methyl-5-cyano-6-hydroxy-nicotinic acid ethyl ester into a 1000mL single-necked flask, add 200mL oxalyl chloride to it, heat ...
example 2
[0572] Example 2: Preparation of intermediate 2-phenyl-4-methyl-5-pyrimidinecarbonyl chloride
[0573] 1) Preparation of 2-phenyl-4-methyl-5-carboxylic acid ethyl ester pyrimidine
[0574]
[0575] Add 18.6g (0.1mol) 2-ethoxymethylene acetoacetate and 15.6g (0.54mol) benzamidine into a three-necked flask containing 200mL ethanol solution, and add 6.8g (0.1mol) sodium ethoxide to it After reacting at room temperature for 5 hours, after TLC monitoring the reaction, the solvent was removed under reduced pressure, the residue was poured into water, and the pH was adjusted to 2 with 20% hydrochloric acid. A large amount of solid precipitated out, filtered by suction, the filter cake was washed with water, and air dried to obtain a white solid 15.6g, yield: 64.5%.
[0576] 2) Preparation of 2-phenyl-4-methyl-5-carboxypyrimidine
[0577]
[0578] Put 10.0g (0.041mol) of intermediate 2-phenyl-4-methyl-5-carboxylic acid ethyl pyrimidine into a 250mL three-necked flask, add 80mL of methanol to...
example 3
[0582] Example 3: Preparation of 1-(3-chloropyridin-2-yl)piperazine hydrochloride
[0583]
[0584] Add 5.0 g (0.027 mol) of N-Boc-piperazine into a 150 mL reaction flask containing 40 mL of N,N-dimethylformamide, and then add 3.7 g (0.027 mol) of potassium carbonate to it, raise the temperature to 40°C, and stir A solution of 4.0 g (0.027 mol) of 2,3-dichloropyridine (commercially available) in N,N-dimethylformamide was added dropwise to it, the addition was completed in 15 minutes, and the reaction was maintained at 80°C for 4 hours. After the reaction was monitored by TLC, the reaction solution was poured into water, a white solid was precipitated out, filtered to obtain 5.5 g of a white solid, the yield: 68.8%.
[0585]
[0586] Put 5.0g (0.017mol) 4-(3-chloropyridin-2-yl)piperazine-1-tert-butyl carbonate into a 250mL single-necked flask, add 50mL tetrahydrofuran, and then add 3.4g (0.034mol ) Concentrated hydrochloric acid, react at room temperature for 4h. After the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com