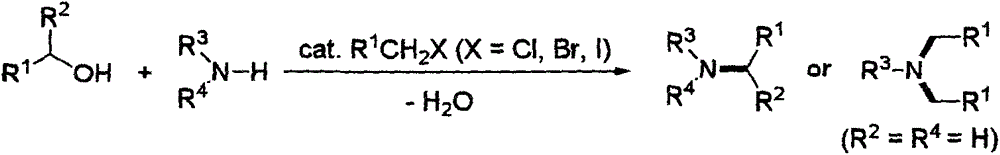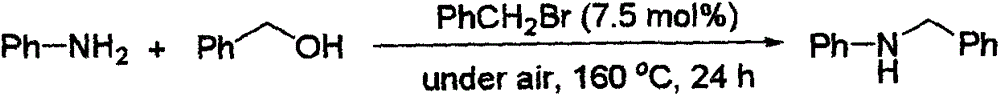Green method for preparing amine derivatives from alcohols and amines
A green technology of amine derivatives, applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems of low reaction selectivity, high price, and many by-products, and achieve wide application prospects, Effects with a wide range of applications and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of N-Benzylaniline from Benzyl Alcohol and Aniline
[0030]
[0031] Benzyl bromide (0.0257g, 7.5mol%), aniline (3mmol, 1.5equiv.) and benzyl alcohol (0.2163g, 2mmol) were successively added into a tubular reactor, sealed directly under air, and then heated under solvent-free conditions Reaction at 160°C for 24h. After the complete reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 82%. 1 H NMR (500MHz, CDCl 3 ): δ7.35-7.30(m, 4H), 7.26-7.24(m, 1H), 7.17-7.14(m, 2H), 6.71-6.68(m, 1H), 6.61-6.59(m, 2H), 4.28 (s, 2H), 3.97(b, 1H). 13 C NMR (125.4MHz, CDCl 3 ): δ148.1, 139.4, 129.2, 128.6, 127.4, 117.5, 112.8, 48.2. MS (EI): m / z (%) 184 (7), 183 (47), 182 (18), 181 (4) , 180(6), 106(18), 104(10), 92(9), 91(100), 89(3), 78(4), 77(23), 65(20), 63(4) , 51(11).
Embodiment 2
[0033] Preparation of N-Benzyl-2-Methylaniline from Benzyl Alcohol and 2-Methylaniline
[0034]
[0035] Add benzyl bromide (0.0257g, 7.5mol%), 2-methylaniline (3mmol, 1.5equiv.) and benzyl alcohol (0.2163g, 2mmol) successively in the tubular reactor, directly seal under the air, then Under solvent conditions, it was heated to 160°C for 24h. After the complete reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 64%. 1 H NMR (500MHz, CDCl 3 ): δ7.44-7.38(m, 4H), 7.34-7.31(m, 1H), 7.16-7.11(m, 2H), 6.72(t, J=7.0Hz, 1H), 6.66(d, J=8.0 Hz, 1H), 4.42(s, 2H), 3.90(b, 1H), 2.21(s, 3H). 13 C NMR (125.4MHz, CDCl 3 ): δ146.1, 139.5, 120.1, 128.6, 127.5, 127.2, 127.1, 121.9, 117.2, 110.0, 48.3, 17.5. MS (EI): m / z (%) 198 (12), 197 (73), 196 (22), 120(15), 118(10), 106(17), 92(9), 91(100), 77(7), 65(16).
Embodiment 3
[0037] Preparation of N-Benzyl-3-Methylaniline from Benzyl Alcohol and 3-Methylaniline
[0038]
[0039]Add benzyl bromide (0.0257g, 7.5mol%), 3-methylaniline (3mmol, 1.5equiv.) and benzyl alcohol (0.2163g, 2mmol) successively in the tubular reactor, directly seal under the air, and then Under solvent conditions, it was heated to 160°C for 24h. After the complete reaction was monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 51%. 1 H NMR (500MHz, CDCl 3 ): δ7.35-7.18(m, 5H), 7.05(t, J=7.5Hz, 1H), 7.53(d, J=7.5Hz, 1H), 6.45-6.42(m, 2H), 4.29(s, 2H), 3.83(b, 1H), 2.25(s, 3H). 13 C NMR (125.4MHz, CDCl 3 ): δ148.2, 139.6, 139.0, 129.1, 128.6, 127.5, 127.1, 118.5, 113.6, 110.0, 48.3, 21.6. MS (EI): m / z (%) 198 (16), 197 (100), 196 (49), 194(5), 180(3), 120(26), 118(8), 92(9), 91(99), 89(3), 77(6), 65(15).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com