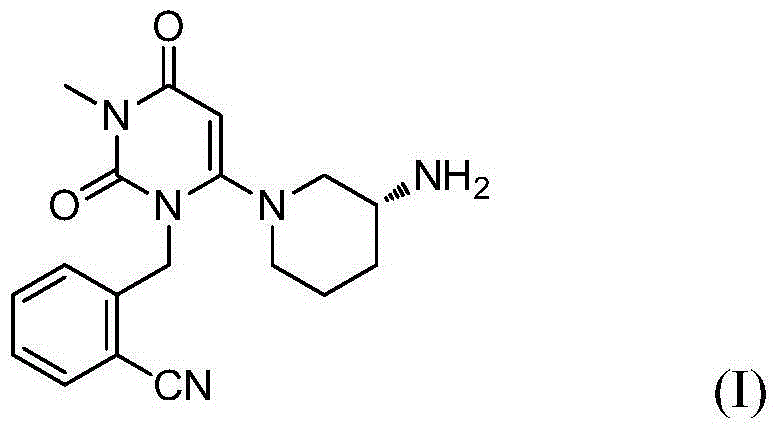
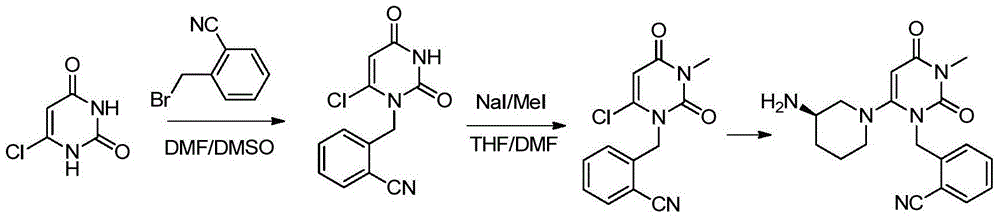
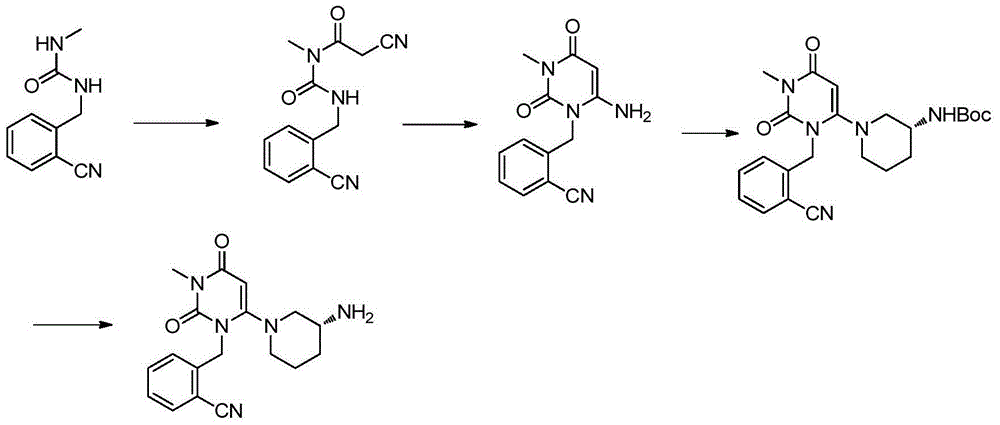
Preparation method of alogliptin
A compound and organic solvent technology, applied in the field of drugs for the treatment of type 2 diabetes, can solve the problems of high price and difficult application of 3-methyl-6-chlorouracil, and achieve low raw material cost, controllable reaction and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] (1) Preparation of 1-methylbarbituric acid (compound of formula III)
[0085] 0.74kg of N-methylurea was added to 5L of glacial acetic acid, the temperature was controlled at 0 to 30°C, 1.4kg of dimethyl malonate was added, heated to 90°C and kept warm until the reaction was complete, acetic acid was recovered under reduced pressure, 3L of 95% ethanol was added, and the mixture was analyzed. Crystallization to obtain 1-methylbarbituric acid (compound of formula III) yellow solid 1.29kg, yield 91%, melting point 130~133℃, 1 H NMR (DMSO-d 6 , 300 MHz): δ=11.31(s, 1H), 3.56(s, 2H), 3.03(s, 3H) ppm.
[0086] (2) Preparation of 1-methyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-ylmethanesulfonate formula (IV)
[0087] 1kg of 1-methylbarbituric acid (compound of formula III) was dissolved in 5L of dichloromethane, added DIPEA 1kg, temperature controlled at 0~30°C, 0.9kg of methanesulfonyl chloride was added dropwise, the temperature was controlled until the reaction was compl...
Embodiment 2
[0095] (1) Preparation of 1-methylbarbituric acid (compound of formula III)
[0096] 0.74kg of N-methylurea was added to 3L of glacial acetic acid, the temperature was controlled at 0 to 30°C, 1.1kg of malonic acid and 2kg of acetic anhydride were added, heated to 90°C and incubated until the reaction was complete, acetic acid was recovered under reduced pressure, and 3L of 95% ethanol was added. , crystallization to obtain 1-methylbarbituric acid (compound of formula III) yellow solid 1.32kg, yield 93%, melting point 132~135℃, 1 H NMR (DMSO-d 6 ,300MHz):δ=11.31(s,1H),3.56(s,2H),3.03(s,3H)ppm.
[0097] (2) Preparation of 1-methyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl p-toluenesulfonate (compound of formula IV)
[0098] 1kg of 1-methylbarbituric acid (compound of formula III) was dissolved in 3L of pyridine, the temperature was controlled at 0~30°C, 1.5kg of p-toluenesulfonyl chloride was added, the temperature was controlled until the reaction was complete, and the reac...
Embodiment 3
[0106] (1) Preparation of 1-methylbarbituric acid (compound of formula III)
[0107] 0.74kg of N-methylurea was added to 3L of absolute ethanol, the temperature was controlled at 0~30°C, 1.6kg of diethyl malonate was added, heated to reflux, incubated until the reaction was complete, cooled to 0~5°C, and crystallized to obtain 1-Methylbarbituric acid (compound of formula III) yellow solid 1.35kg, yield 95%, melting point 131~133℃, 1 H NMR (DMSO-d 6 ,300MHz):δ=11.31(s,1H),3.56(s,2H),3.03(s,3H)ppm.
[0108] (2) Preparation of 1-methyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl trifluoromethanesulfonate (compound of formula IV)
[0109] 0.1kg of 1-methylbarbituric acid (compound of formula III), dissolved in 0.5L of 2-methyltetrahydrofuran, temperature controlled at 0~30℃, added with 0.1kg of triethylamine, and added dropwise with 0.2kg of trifluoromethanesulfonic anhydride , control the temperature until the reaction is complete, add the reaction solution to 0.5L ice water, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com