Platinum (II) alkyne complex and application thereof
A technology of complexes and compounds, applied in the field of nonlinear optical materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of fluorene-bipyridine platinum (II) chloride complex (compound M-1)
[0023] Reaction formula:
[0024]
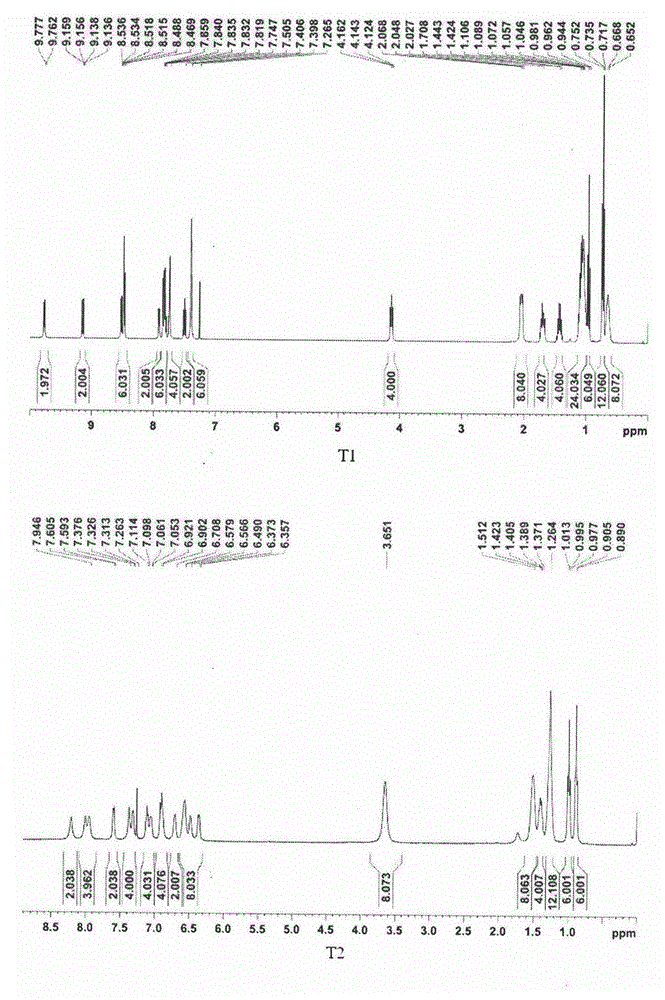
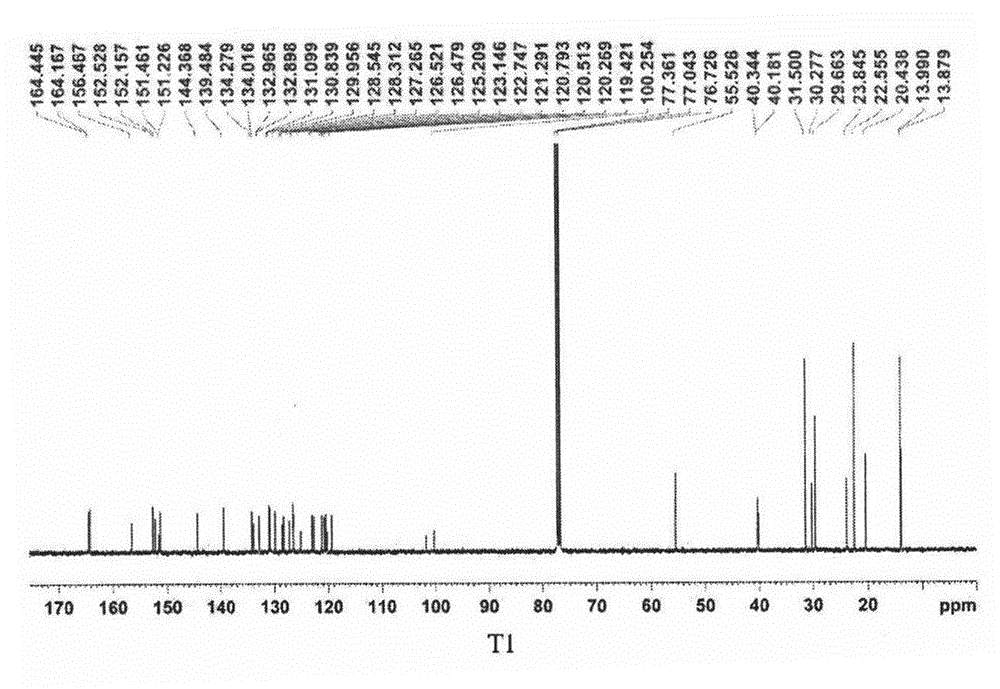
[0025] Add (224mg, 0.534mmol) platinum (II) chlorodimethylsulfoxide complex 2 and (438mg, 0.534mmol) 4,4'-bis(9,9-dihexylfluorenyl) to a 250mL three-necked flask Bipyridine (compound C-1), and then add 60 mL of acetonitrile and 100 mL of dichloromethane respectively. Heat to reflux for 6-8 hours, cool down to room temperature after the reaction, and spin off the solvent under reduced pressure. The obtained crude product was purified by column chromatography, and the developing solvent was dichloromethane. 290 mg of yellow solid were obtained, yield 87.2%. 1 H NMR (400MHz, CDCl 3 ): δ9.73(m, 2H), 8.22(s, 2H), 7.83(d, J=7.8Hz, 2H), 7.73(d, J=2.5Hz, 6H), 7.64(s, 2H), 7.33 (m, 6H), 2.01-1.97 (m, 8H), 1.05-0.96 (m, 24H), 0.67 (t, J=6.9Hz, 12H,), 0.60-0.56 (m, 8H). 13 C NMR (400MHz, CDCl 3 )δ (ppm): 153.8, 150.5, 148.3, 147.8, 143.1...
Embodiment 2
[0026] Embodiment 2: the synthesis of compound T-1
[0027] Reaction formula:
[0028]
[0029]In the three-necked flask of 250mL, add (145mg, 0.134mmol) compound M-1, (92mg, 0.333mmol) N-butyl-6-ethynyl naphthalimide (compound N-Bu-NI≡) and ( 6mg, 0.003mmol) of cuprous iodide, and then added 50mL of anhydrous diisopropylamine and 90mL of anhydrous dichloromethane. Heating to reflux under nitrogen protection for 20-24 hours, after the reaction was completed, it was cooled to room temperature, and the solvent was rotated off under reduced pressure. The obtained crude product was purified by column chromatography, and the developing solvent was dichloromethane:petroleum ether=1:1. 122 mg of a red solid was obtained, yield 58.2%. 1 H NMR (400MHz, CDCl 3 ): δppm 9.87(d, J=5.9Hz, 2H), 9.21(d, J=8.3Hz, 2H), 8.58-8.49(m, 6H), 8.00-7.75(m, 12H), 7.56(t, J =7.8Hz, 2H), 7.43(d, J=2.9Hz, 6H), 4.21(t, J=7.6Hz, 4H), 2.09-2.05(dd, J=10.7Hz, J=5.4Hz, 8H), 1.79-1.72(m, 4H), 1.53-1.4...
Embodiment 3
[0030] Embodiment 3: the synthesis of carbazole-bipyridine platinum (II) chloride complex (compound M-2)
[0031] Reaction formula:
[0032]
[0033] The process was the same as in Example 1, except that the reactant C-1 was replaced with 4,4'-bis(N-hexylcarbazolyl)bipyridine (compound C-2).
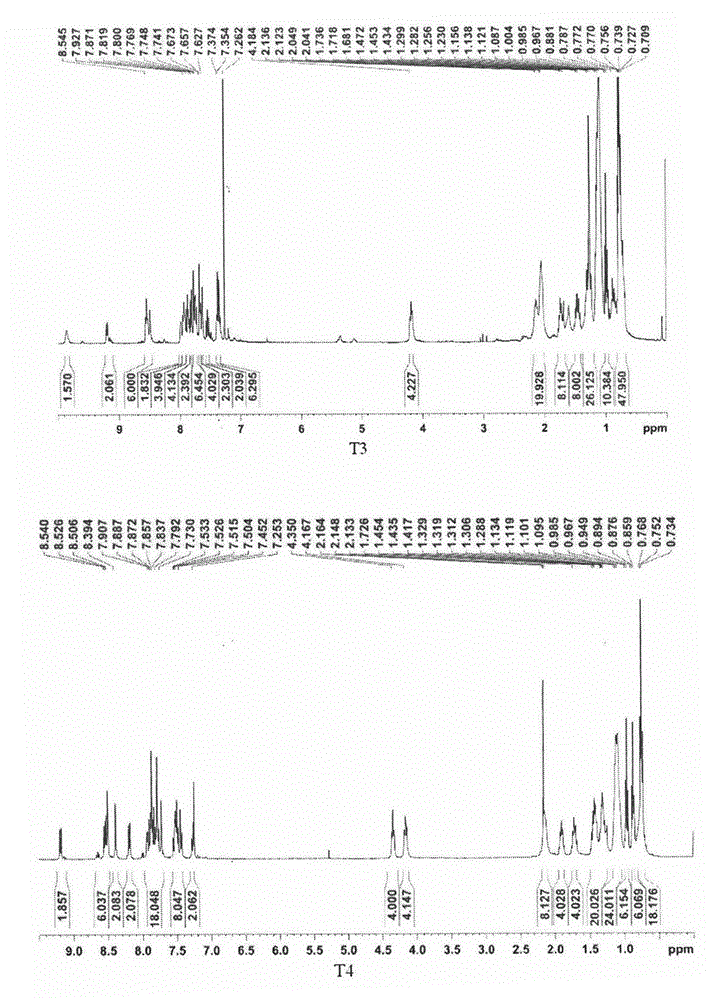
[0034] The obtained crude product was purified by column chromatography, and the developing solvent was dichloromethane. A yellow solid was obtained in a yield of 91.2%. 1 H NMR (400MHz, CDCl 3 ): δ8.91(s, 2H), 8.82-8.81(d, J=5.0Hz, 2H), 8.58(s, 2H), 8.24-8.22(d, J=3.8Hz, 2H), 7.94-7.91( dd, J=4.3Hz, 2H), 7.72-7.70(dd, J=2.5Hz, 2H), 7.54-7.43(m, 6H), 7.33-7.29(t, J=3.7Hz, 2H), 4.33-4.29 (t, J=4.0Hz, 4H), 1.93-1.86(m, 4H), 1.41-1.28(m, 12H), 1.07-1.05(m, 16H), 0.91-0.87(t, J=3.4Hz, 12H ). 13 C NMR (400MHz, CDCl 3 ):156.82,150.21,149.60,141.01,140.97,128.90,126.11,124.95,123.50,122.95,121.61,119.31,119.25,119.10,109.16,109.02,43.27,31.61,29.00,27.01,22.60,14.08.Elemental analysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com