Aryl five-membered heterocyclic substituted quinoline platinum (II) complex, preparation method thereof and application
A five-membered heterocycle, quinoline platinum technology, applied in the field of aromatic five-membered heterocycle substituted quinoline platinum complexes, can solve the problem of reducing the luminous quantum efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis of complex [2(Thip-2-Ph)4Me]Pt(acac)
[0039]
[0040] (1) 4-Methylquinoline-2(1 H )-ketone synthesis
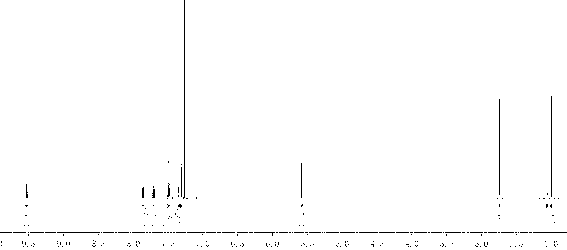
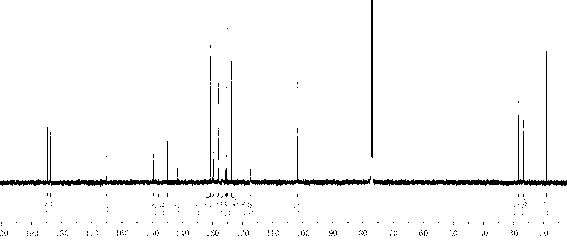
[0041] Add 40mL of concentrated sulfuric acid into a three-necked flask with a stirring bar, and gradually add N -Acetoacetanilide 17.7g, add 10mL of concentrated sulfuric acid after adding, heat up to 80°C for 10min, then carefully pour into 400mL of ice water after completion, a large amount of white precipitate precipitates, vacuum filter, wash with water until neutral, at 30°C Vacuum drying gave a white solid with a yield of 87%. Colorless rod-shaped crystals were precipitated by ethanol recrystallization. 1 H NMR (600MHz, CDCl 3 ) δ(ppm): 11.95(br; 1H); 7.70(dd; J =1.20Hz; J =7.20Hz; 1H); 7.52(dt; J =1.20Hz; J =7.20Hz; 1H); 7.42(dd; J =1.20Hz; J =7.20Hz; 1H); 7.26(dt; J =1.20Hz; J =7.20Hz; 1H); 6.61(q; J =1.20Hz; 1H); 2.53(d; J =1.20Hz; 3H).
[0042] (2) Synthesis of 2-chloro-4-methylquinoline
[0043] Take 4-methylqu...
Embodiment 2
[0053] Embodiment 2: the synthesis of complex [2(Thip-2-Ph)3F4Me]Pt(acac)
[0054]
[0055] (1) 3-bromo-4-methylquinoline-2(1 H )-ketone synthesis
[0056] Get the synthetic 4-methylquinoline-2(1 of embodiment 1 H )-ketone 7.0g, bromine 7.04g, anhydrous acetic acid 100mL, anhydrous DMF 45mL. First dissolve 4-methylquinoline-2 (1 H )-ketone solution in anhydrous acetic acid was added to a three-necked flask with a stirrer, protected from light, then heated to 65°C and stirred, then quickly added in anhydrous acetic acid solution of bromine, and after the addition was completed, 45 mL of anhydrous DMF, the reaction solution turns into a brown transparent solution, stirred and reacted at 65°C for 5 hours, the reaction solution turned into light yellow and transparent, stopped heating, and after slow cooling, a large amount of white crystals precipitated, the reaction product was poured into 600mL of water, and a large amount of white flocs were precipitated A white filter ...
Embodiment 3
[0067] Example 3: Synthesis of complex [2(Thip-2-Ph)3,7DiF4Me]Pt(acac)
[0068]
[0069] (1) 3,7-dibromo-4-methylquinoline-2(1 H )-ketone synthesis
[0070] Get the synthetic 4-methylquinoline-2(1 of embodiment 1 H )-ketone 7.0g, bromine 14.07g, anhydrous acetic acid 100mL, anhydrous DMF 45mL. First dissolve 4-methylquinoline-2 (1 H Add the anhydrous acetic acid solution of )-ketone into a three-necked flask with a stirrer, heat to 65°C in the dark and stir, then quickly add the bromine anhydrous acetic acid solution dropwise, and then add 45mL of anhydrous DMF to the reaction solution Turn into a brown transparent liquid, stir and react at 65°C for 10h, the reaction liquid becomes light yellow and transparent, stop heating, cool slowly, a large amount of white crystals are precipitated, the reaction product is poured into 600mL water, a large amount of white flocculent solids are precipitated, pumped A white filter cake was obtained by filtration, washed with water thr...
PUM
| Property | Measurement | Unit |
|---|---|---|
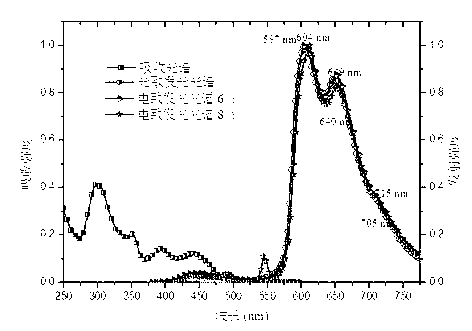
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com