Application of dermastin a in the preparation of preparations for suppressing immune rejection of organ transplantation
A technology of immune rejection and dermatocin, applied in medical preparations containing active ingredients, gene therapy, peptide/protein components, etc., can solve the problem of low long-term survival rate, achieve the goal of improving survival rate and inhibiting immune rejection Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: Amplification of HA gene (accession number X74303)
[0012] Total RNA was extracted from the first-stage larvae of Psofida, and cDNA was synthesized by reverse transcription. Using cDNA as a template, the upstream and downstream primers are F1 5'-ATGCTGAAGTTTGTTATTTTATTGTGC-3' (SEQ ID NO. 1), R1 5'-TA GGATCC TTAAATAGATTC TGCATTACTGAC-3ˊ (SEQ ID NO. 2) (the underlined part is the restriction site of BamH I), the amplified fragment size is 771 bp. In order to obtain the secreted protein of the HA gene, a signal peptide sequence ATGGAGACAGACACACTCCTGCTATGGGTACTGCTGCTCTGGGTTCCAGGTTCCACTGGTGACGCGGCC (SEQ ID NO.3) was added to the starting site of the HA gene by overlapping PCR method. At the same time, a pair of primers with enzyme cutting sites were designed: F2: 5ˊ-GT GAATTC ATGGAGACAGACACACTCC-3ˊ (SEQ ID NO.4) (the underlined part is the restriction site of EcoR I), R1: 5ˊ-TA GGATCC TTAAAT AGAT TCT GCATTACTGAC-3' (SEQ ID NO.5) (the underlined part is the ...
Embodiment 2
[0013] Example 2: Construction of eukaryotic expression vector pEF1α-HA-AcGFP
[0014] The PCR product of the HA gene and the eukaryotic expression vector pEF1α-IRES-AcGFP (purchased by Treasure Bioengineering (Dalian) Co., Ltd.) were purified and recovered, and simultaneously digested with EcoR I and BamH I, and the digested product was digested with 1% agarose Gel electrophoresis, gel cutting and recovery were mixed at a molar ratio of 3:1, and ligated with T4 DNase for 3 h. The reaction product was transformed into DH5α competent cells, screened with a kanamycin plate, picked 8 single colonies, inoculated in 3 mL LB medium containing kanamycin, and cultured overnight at 37 °C with shaking. F3: AGCGGGCGGGTGAGTCA (SEQ ID NO.6) and R3: GATACTTGCCAAGGAAAATC (SEQ ID NO.7) were designed on the HA gene fragment and plasmid respectively
[0015] Primers, perform PCR on the cultured bacterial solution overnight, take the bacterial solution that shows the correct size of the amplifi...
Embodiment 3
[0016] Embodiment 3: Establishment of HA transgenic animal model
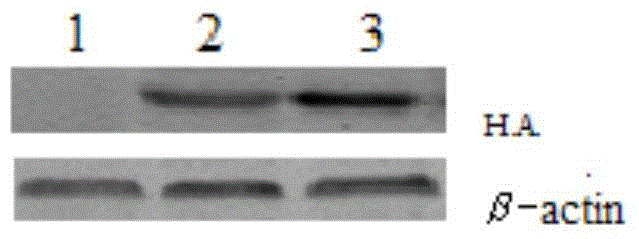
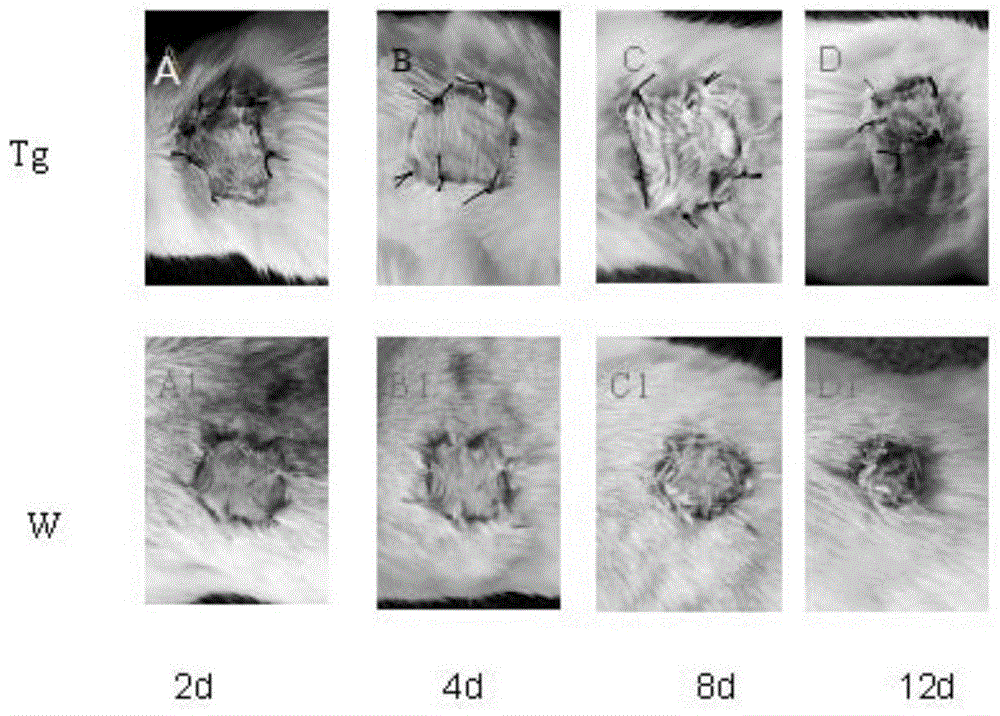
[0017] HA transgenic mice were prepared by microinjection technology (assisted by Guangzhou Saiye Biotech Co., Ltd.). figure 2 It was shown that HA transgenic mice successfully expressed HA protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com