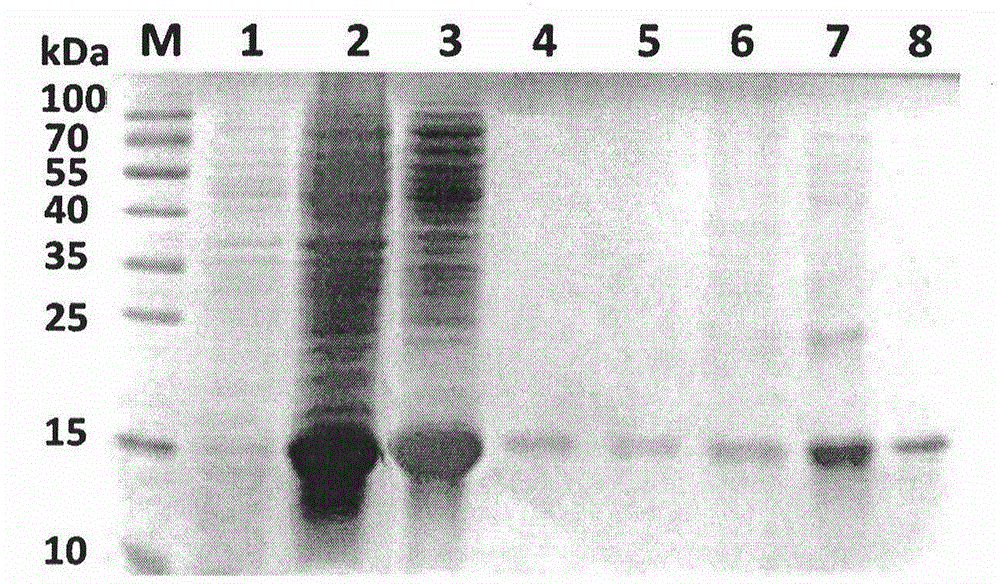
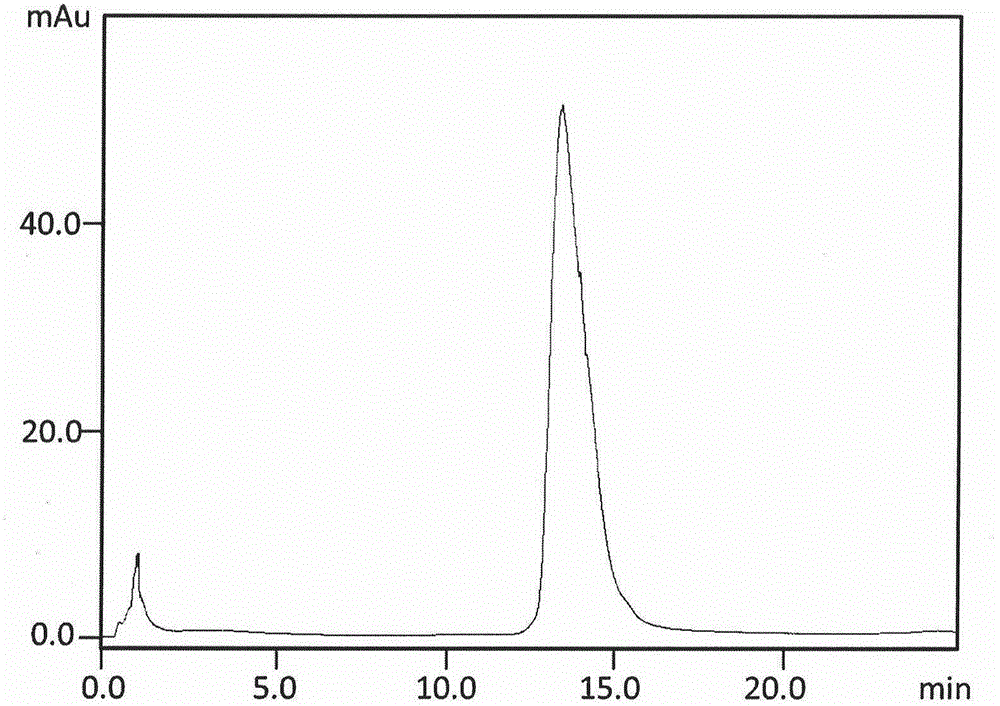
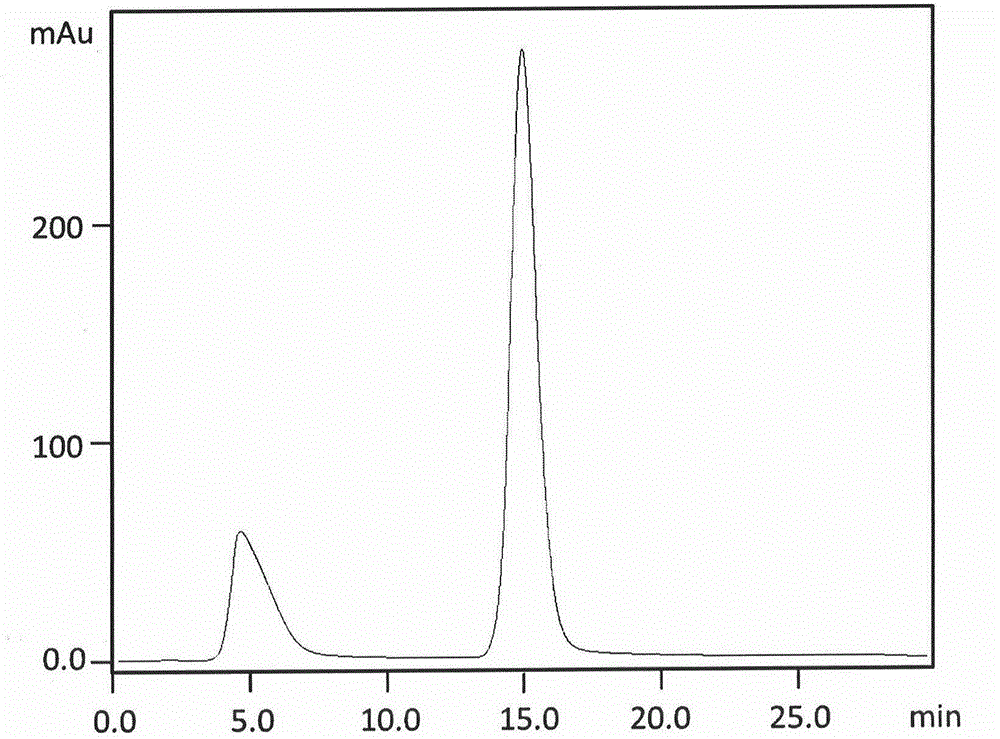
Purification and renaturation method of Notch ligand Delta-like1 fusion protein
A fusion protein and ligand technology, applied in the field of denatured protein renaturation, can solve the problems of cumbersome process, high cost of stationary phase, unsuitable for mass production, etc., and achieve the effect of overcoming high cost, short purification process cycle and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for purification and renaturation of Notch ligand Delta-like1 fusion protein, comprising the following steps:
[0031] 1) The inclusion bodies containing HD1R were washed with washing buffer several times, centrifuged, and dissolved in strong denaturant buffer to obtain crudely separated HD1R protein;
[0032] 2) Directly inject the denatured extract containing human HD1R protein into a column equilibrated with mobile phase A (20mmol / LPBS, pH8.0), and then use mobile phase B containing 20mmol / LPBS, 1mol / LNaCl, pH7.5 Isocratic elution, collecting target peak chromatographic fractions to obtain renatured and purified HD1R chromatographic fractions;
[0033] 3) Directly inject the collected chromatographic fraction of the target peak, further desalt with a chromatographic column equilibrated with 20mmol / LPBS mobile phase, and continue elution for 30min at a flow rate of 1.5mL / min to obtain an active chromatographic fraction with a higher degree of renaturation and...
Embodiment 2
[0035] A method for purification and renaturation of Notch ligand Delta-like1 fusion protein, comprising the following steps:
[0036] 1) The inclusion bodies containing HD1R were washed with washing buffer several times, centrifuged, and dissolved in strong denaturant buffer to obtain crudely separated HD1R protein;
[0037] 2) Directly inject the denatured extract containing human HD1R into a chromatographic column equilibrated with mobile phase A (40mmol / LPBS, or 20-40mmol / LTris, pH7.6), and then use a column containing 40mmol / LTris, 1mol / LNaCl, pH8 .0 mobile phase B isocratic elution, collect the chromatographic fraction of the target peak, and obtain the chromatographic fraction of HD1R after renaturation and purification;
[0038] 3) Directly inject the collected chromatographic fraction of the target peak, further desalt with a chromatographic column equilibrated with 20mmol / LPBS mobile phase, and continue elution for 30min at a flow rate of 1.5mL / min to obtain an activ...
Embodiment 3
[0040] A method for purification and renaturation of Notch ligand Delta-like1 fusion protein, comprising the following steps:
[0041] 1) The inclusion bodies containing HD1R were washed with washing buffer several times, centrifuged, and dissolved in strong denaturant buffer to obtain crudely separated HD1R protein;
[0042] 2) Directly inject the denatured extract containing human HD1R protein into the chromatographic column equilibrated with mobile phase A (30mmol / LPBS, pH7.7), and then use the mobile phase B line containing 30mmol / LPBS, 1mol / LNaCl pH7.8 Isocratic elution, collecting target peak chromatographic fractions to obtain renatured and purified HD1R chromatographic fractions;
[0043] 3) Directly inject the collected chromatographic fraction of the target peak, use a chromatographic column equilibrated with 20mmol / LPBS mobile phase for further desalting, continue elution for 30min, flow rate 0.5mL / min, and obtain an active chromatographic fraction with a higher deg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com