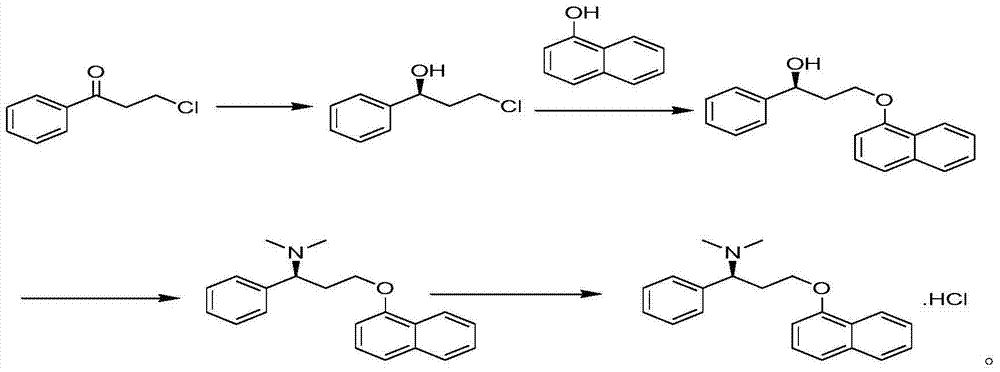
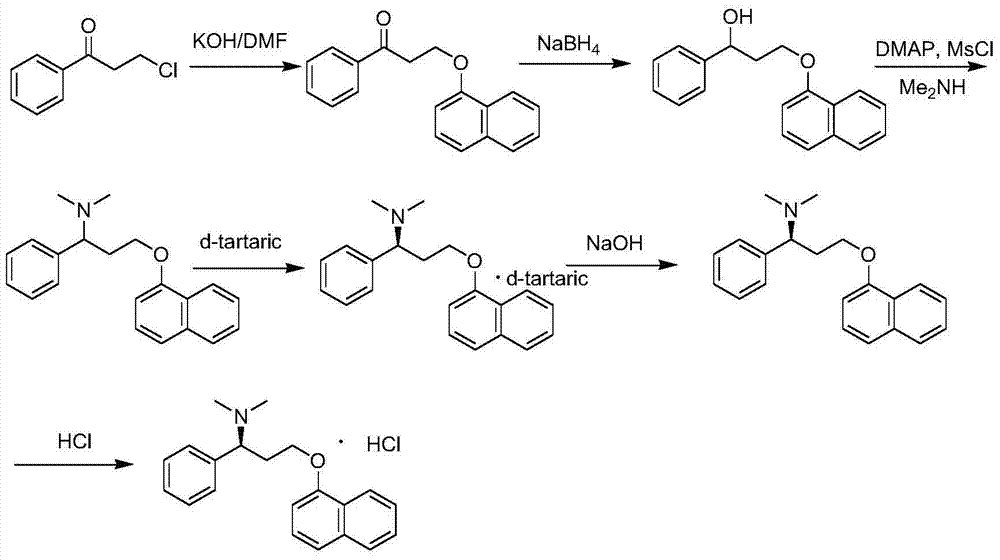
New crystal form of dapoxetine hydrochloride as well as preparation method and application thereof
A technology of dapoxetine hydrochloride and dapoxetine, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., can solve unstable preparations, poor stability of crystal form A and crystal form B, The problems of high manufacturing cost and use cost, etc., achieve the effect of good stability, easy post-processing, and shortened drying time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Take 1g of dapoxetine and add it into a clean and dry three-necked reaction flask, add 10ml of ethyl acetate, stir at 25±2°C until the solid dissolves completely, filter, and slowly pour into the obtained filtrate an equimolar amount of dapoxetine Hydrogen chloride gas, lower the temperature to -5±2°C, stir and crystallize for 1 hour, filter, and vacuum-dry the obtained material at 50±2°C to obtain 1.03g of white solid powder, which is the new crystal form C of dapoxetine hydrochloride. was 92.3%. X-ray powder diffraction pattern see attached figure 1 .
[0071] The X-ray powder diffraction characteristic absorption peak of the new crystal form C of table 1 embodiment 1 gained dapoxetine hydrochloride
[0072] 2θ
[0073] 24.28
Embodiment 2
[0075] Take 1g of dapoxetine and add it to a clean and dry three-necked reaction flask, add 10ml of ethyl propionate, stir at 25±2°C until the solid dissolves completely, filter, and slowly pour an equimolar amount of dapoxetine into the obtained filtrate Hydrogen chloride gas, cooled to -5 ± 2 ° C, stirred and crystallized for 1 hour, filtered, and the obtained material was vacuum-dried at 50 ± 2 ° C to obtain 0.98 g of white solid powder, which was the new crystal form C of dapoxetine hydrochloride. The rate is 87.4%. The X-ray powder diffraction pattern is within the error range and figure 1 unanimous.
Embodiment 3
[0077] Take 1g of dapoxetine and add it into a clean and dry three-necked reaction flask, add 10ml of methyl formate, stir at 25±2°C until the solid dissolves completely, filter, and slowly pour into the obtained filtrate an equimolar amount of dapoxetine Hydrogen chloride gas, lower the temperature to -5±2°C, stir and crystallize for 1 hour, filter, and vacuum-dry the obtained material at 50±2°C to obtain 1.02g of white solid powder, which is the new crystal form C of dapoxetine hydrochloride. was 91.4%. The X-ray powder diffraction pattern is within the error range and figure 1 unanimous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com