Preparation method of 2-chloro-5-trichloromethylpyridine
A technology of trichloromethylpyridine and picoline, which is applied in the field of chemical compounds, can solve the problems of many reaction steps, difficult separation, pollution, etc., and achieve the effects of mild reaction conditions, sufficient vapor-liquid contact, and reduced side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Take 500 g of 3-picoline and dissolve in 2000 g of chloroform, then add 10 g of phosphorus pentachloride and 1 g of azobisisobutyronitrile, stir and mix to form a homogeneous feed solution.
[0022] Feed liquid is injected into the first plate of the microreactor with a speed of 100g / h with a horizontal flow pump, and the reaction temperature is kept at 90°C, and chlorine gas is passed into the second plate of the microreactor at a speed of 2L / min. After the materials are mixed on the mixing plate, they react at a constant temperature, continuously feed and discharge materials, collect the reaction liquid, reflux through nitrogen to drive away chlorine and hydrogen chloride, separate and recover the chlorination transition products and put them in the raw materials to continue the reaction.
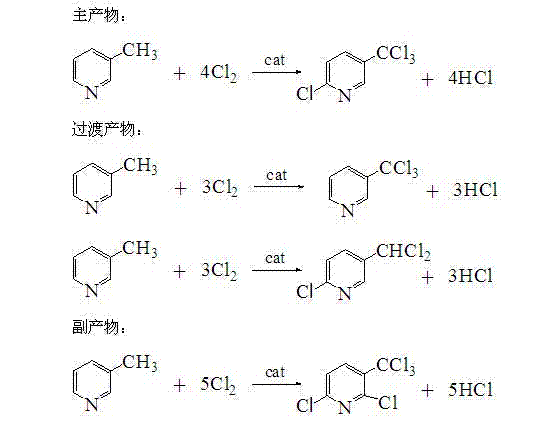
[0023] Sampling liquid spectrum analysis, the analysis results were: 9.2% of 3-trichloromethylpyridine, 5.1% of 2-chloro-5-dichloromethylpyridine, 75.20% of 2-chloro-5-trichlorometh...
Embodiment 2
[0026] Take 500 g of 3-picoline and dissolve in 3000 g of carbon tetrachloride, then add 20 g of phosphorus pentachloride and 2 g of azobisisobutyronitrile, stir and mix to form a homogeneous phase. Use a horizontal flow pump to pump the feed liquid into the microreactor at a speed of 250g / h, keep the temperature at 110°C, and pass chlorine gas into the microreactor at a speed of 6L / min. After the materials are mixed on the mixing plate, they will react at a constant temperature and feed continuously. Discharge, collect the reaction liquid, reflux through nitrogen to catch chlorine and hydrogen chloride, then remove the solvent, sample liquid analysis content: 7.5% of 3-trichloromethylpyridine, 4.1% of 2-chloro-5-dichloromethylpyridine, 73.4% of 2-chloro-5-trichloromethylpyridine, 6.5% of 2,6-dichloro-5-trichloromethylpyridine; high-vacuum rectification of desolvation feed liquid to obtain 2-chloro-5-trichloromethylpyridine The yield of pyridine was 72.36%.
Embodiment 3
[0028] Take 500 g of 3-picoline and dissolve in 4000 g of chloroform, then add 20 g of phosphorus pentachloride and 2 g of benzoyl peroxide, stir and mix to form a homogeneous phase. Use a horizontal flow pump to pump the feed liquid into the microreactor at a speed of 300g / h, keep the temperature at 100°C, and pass chlorine gas into the microreactor at a speed of 8L / min. After the materials are mixed on the mixing plate, they will react at a constant temperature and feed continuously. Discharging, collecting the reaction liquid, refluxing the nitrogen gas to catch chlorine and hydrogen chloride, and then removing the solvent, the content of the sample liquid spectrum analysis: 10.2% of 3-trichloromethylpyridine, 4.1% of 2-chloro-5-dichloromethylpyridine, 75.8% of 2-chloro-5-trichloromethylpyridine, 4.8% of 2,6-dichloro-5-trichloromethylpyridine; high vacuum distillation of desolvation feed liquid to obtain 2-chloro-5-trichloromethylpyridine The yield of pyridine was 76.60%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com