Drug composition reducing in-vivo and in-vitro toxicity of nano drug delivery material and preparation method thereof
A technology of drug complexes and material bodies, applied in the biological field, can solve problems such as the weakening of autophagy ability, and achieve the effect of enhancing safety and reducing toxicity in vivo and in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1. Dendrimers are cytotoxic to human hepatocytes
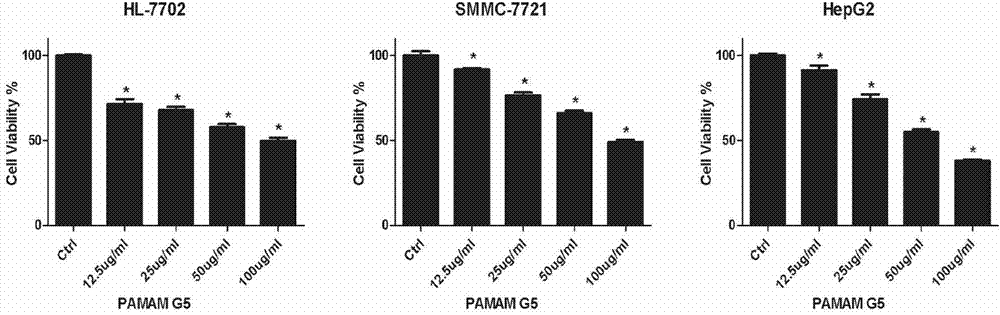
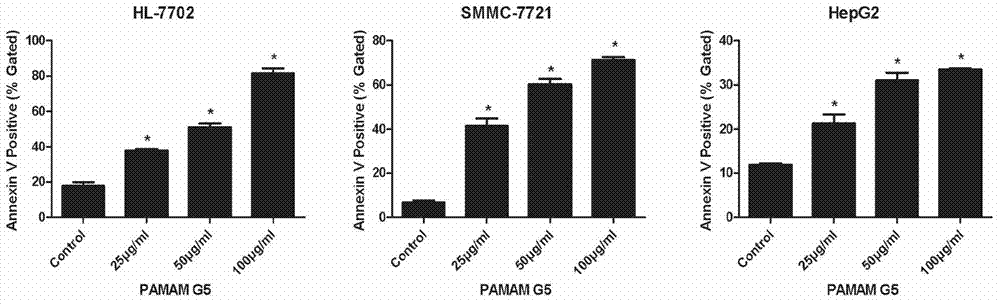
[0042] Normal human liver cells HL7702, liver cancer cells SMMC7721, and HepG2 were treated with different concentrations of PAMAM dendriemrs (12.5 μg / ml-100 μg / ml) for 24 hours, and the relative cell viability was measured by MTT method with untreated cells as negative control. The experimental results are as follows: figure 1 Shown; Annexin V / PI was used to stain cells, and flow cytometry was used to detect cell apoptosis. The experimental results were as follows figure 2 Shown; JC-1 was used to stain the next report, and flow cytometry was used to detect the collapse of mitochondrial membrane potential. The experimental results are as follows image 3 shown.
Embodiment 2
[0043] Example 2. Dendrimers Induce Autophagy in Human Hepatocytes
[0044] Human normal liver cells HL7702, liver cancer cells SMMC7721, and HepG2 were treated with 100 μg / ml dendrimers for 24 hours, then embedded in paraffin, sectioned, and stained, and the submicroscopic structure of the cells was observed under a transmission electron microscope. The results are as follows: Figure 4 As shown, there are a large number of typical double-membrane structure autophagosomes in the cells of the administration group, but not found in the control group.
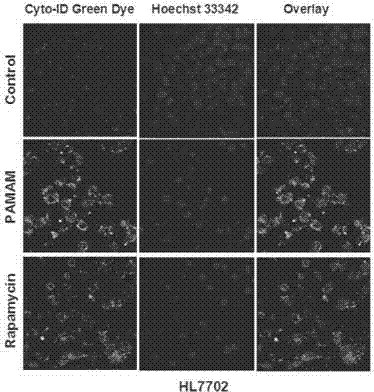
[0045] Normal human liver cells HL7702, liver cancer cells SMMC7721, and HepG2 were treated with 100 μg / ml dendrimers for 24 hours, stained with Cyto-ID autophagy detection fluorescent dye, and observed under a laser confocal microscope. The results are as follows: Figure 5 As shown, obvious green fluorescence can be observed in the cells of the administration group and the cells of the rapamycin effect group, while the green fl...
Embodiment 3
[0047] Example 3, 3-methyladenine inhibits autophagy and weakens hepatocyte growth inhibition caused by dendrimers
[0048] Human normal liver cells HL7702 were treated with different concentrations of PAMAM dendriemrs (12.5μg / ml-100μg / ml) for 24 hours, and 3-methyladenine was added to treat the cells 3 hours before administration, and the cell viability of each group was measured by MTT method after 24 hours . Experimental results such as Figure 7 As shown, 3-methyladenine pretreatment can significantly attenuate the growth inhibition of hepatocytes induced by dendrimers. The cells collected by centrifugation were washed once with PBS, and the cells were lysed with RIPA kits. After quantification, 20 μg of protein in each lane was electrophoresed and then transferred to PVDF membranes. Blocked with 5% skimmed milk for 1 h, LC3b and β-actin antibody, incubated at 4°C for 12h. After washing the membrane with TBST, the secondary antibody was added to incubate at room tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com