Medicinal composition of paclitaxel
A composition and technology of paclitaxel, which are applied in the directions of drug combination, anti-tumor drug, powder delivery, etc., can solve the problems of large particle size and complicated process of paclitaxel freeze-dried powder, and achieve the advantages of avoiding adverse reactions, simple preparation process and blood stability. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1-7
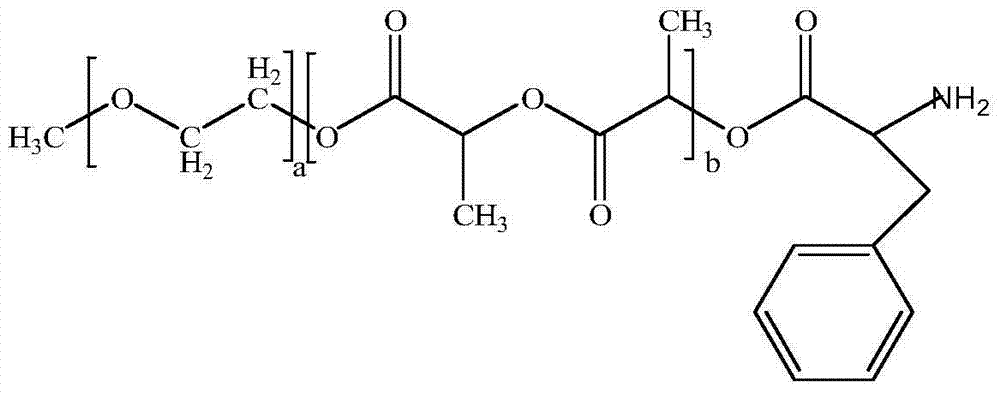
[0032] A pharmaceutical composition of paclitaxel, which is a medicament composed of the following components by weight:
[0033] Paclitaxel 2.87~27.66 parts,
[0034] mPEG-PLA-Phenylalanine 100 parts.
[0035] The preparation method of composition among the present invention comprises the following steps:
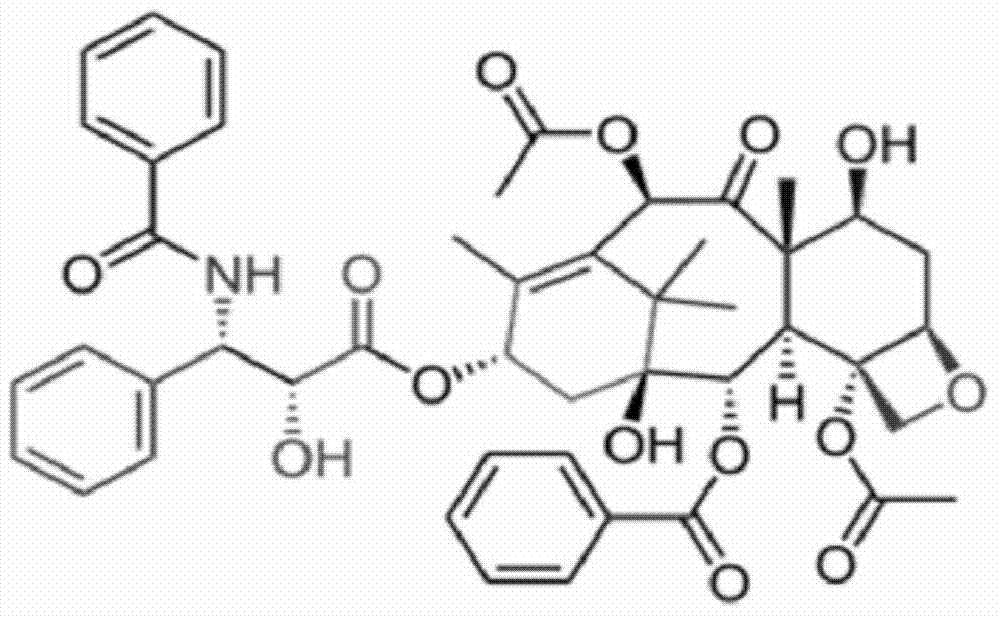
[0036]① Weigh raw materials (paclitaxel, mPEG-PLA-phenylalanine) according to different feeding ratios. Among them, paclitaxel (CAS33069-62-4) is produced by Xi'an Ruilin Biotechnology Co., Ltd., with a purity greater than 95%. preparation;
[0037] ②Put the above-mentioned raw materials into a container, and add organic solvents such as ethanol or acetonitrile at a temperature of 15-45°C until they are completely dissolved. The dissolving process can adopt means such as stirring or shaking.
[0038] ③ Rotate the above solution at 30-50°C for 2 hours until the organic solvent evaporates to dryness. Then vacuum drying at 10-40° C. for > 12 hours to remove residual org...
Embodiment 8
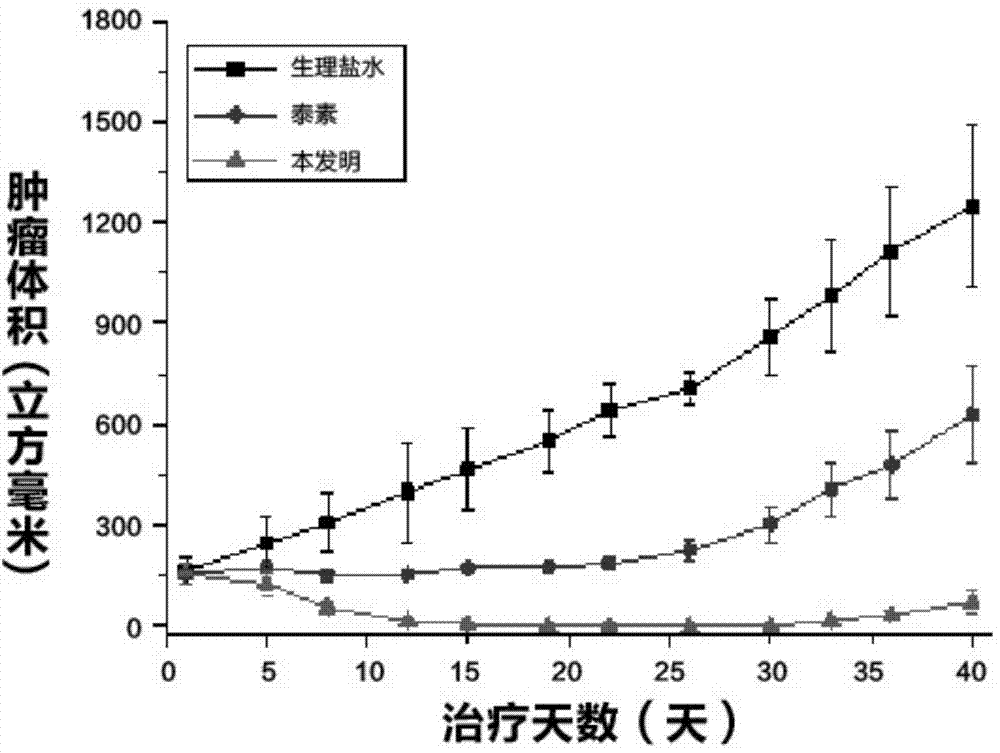
[0057] Example 8 Tumor Inhibition Experiment
[0058] Using normal saline, taxol (commercially available paclitaxel injection) and the paclitaxel micelle solution obtained in Example 3, the inhibition test of human MCF-7 breast cancer xenograft tumor was carried out on nude mice. The route of administration is intravenous administration, administered once every three days, and the concentration of taxol and paclitaxel of the present invention is 20mg / kg equally. The tumor volume of nude mice was measured every day, and the results were as follows: figure 1 shown. The results show that: the tumor volume of the nude mice instilled with normal saline increases rapidly; the growth rate of the tumor volume of the nude mice instilled with Taxol is controlled to a certain extent, but the tumor volume still increases; the tumor volume of the nude mice instilled with the present invention is controlled and gradually decreases. Small. It is proved that the present invention has remar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com