Imidazoline corrosion inhibitor, synthetic method and application thereof
The technology of imidazoline and synthesis method is applied in the field of imidazoline corrosion inhibitor and synthesis, and can solve the problems of pitting corrosion, iron corrosion, safety of boiler system, hidden dangers left by stable operation, etc., and achieves simple usage and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment provides an imidazoline corrosion inhibitor, which is obtained by reacting rosin with diethylenetriamine to obtain an imidazoline intermediate, and then reacting with N,N-dimethylacetamide to obtain rosin -Imidazolium-N, N-dimethylacetamidine, finally obtained by reacting with carbon dioxide.
[0042] The present embodiment also provides the synthetic method of above-mentioned imidazoline corrosion inhibitor, comprises the following steps:
[0043] Step 1: Reaction of rosin and diethylenetriamine to obtain an imidazoline intermediate, the chemical reaction formula is as follows,
[0044] .
[0045] Step 1 specifically includes the following steps including the following steps,
[0046] (1) Mix rosin (58.0795g, 0.1731moL) and xylene at a mass ratio of 1:9, and add it to a three-necked flask equipped with a water separator, a reflux condenser, and a thermometer;
[0047] (2) Heat to 100°C to completely dissolve the rosin in xylene, and start to add di...
Embodiment 2
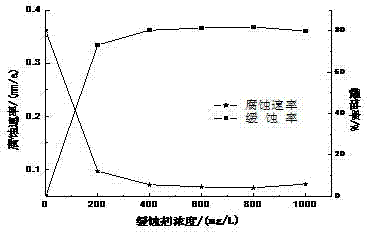
[0062] This embodiment provides an application of the above-mentioned imidazoline corrosion inhibitor: the concentration range in use is 200-400 mg / L.
[0063] The following are confirmed by specific experiments:
[0064] 1. Synthesis of corrosion inhibitors
[0065] Synthesize the corrosion inhibitor according to the synthesis method of the corrosion inhibitor, that is, use 58.0795g (0.1731mol) of rosin and 25.0000g (0.2423mol) of diethylenetriamine as raw materials, synthesize the rosin-imidazoline intermediate by solvent method, and then Using 65.0000g (0.7496mol) of rosin-imidazoline intermediate and 56.1759g (0.2496mol) of N,N-dimethylacetamide as raw materials to synthesize rosin-imidazoline-N,N-dimethylacetamide by direct synthesis Finally, react the synthesized rosin-imidazoline-N,N-dimethylacetamidine with carbon dioxide under certain conditions to generate rosin-imidazoline-acetamidine bicarbonate. Under the condition that the corrosive medium is alcohol-containing...
Embodiment 3
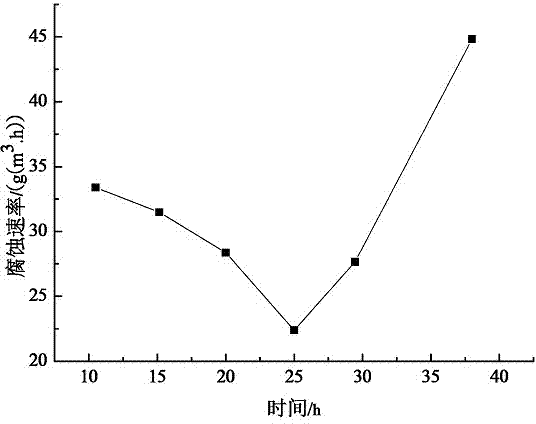
[0097] This embodiment provides an application of the above-mentioned imidazoline corrosion inhibitor: after every 25 hours in use, a new imidazoline corrosion inhibitor needs to be replaced.
[0098] The following are confirmed by specific experiments:
[0099] 1. Synthesis of corrosion inhibitors
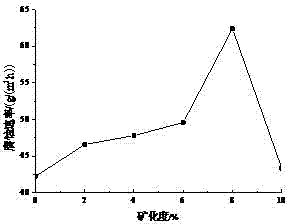
[0100] Synthesize novel imidazoline corrosion inhibitor according to the method for synthesizing corrosion inhibitor in embodiment 2, then when corrosion inhibitor consumption is 700mg / L, corrosion medium is that mass fraction is 3%NaCl and 1% hydrochloric acid solution, and corrosion temperature is 80 ℃, the corrosion test was carried out to investigate the influence of corrosion time on the corrosion inhibition effect of corrosion inhibitors.
[0101] 2. Static hanging test as in Example 2.
[0102] 3. Experimental results
[0103] like figure 2 As shown, before the corrosion time is 25 h, the corrosion rate gradually decreases with the prolongation of the corrosion time, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com