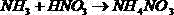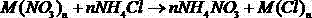Method for removing nitric acid and nitrates from high-level radioactive liquid waste
A high-level waste liquid and nitrate technology, which is applied in radioactive purification, nuclear engineering, etc., can solve the problems of high energy consumption and high requirements for electrode corrosion resistance, and achieve the effects of reducing the risk of explosion, high safety and stability, and easy implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1. Synthesize urea-formaldehyde resin (UF resin) with a molar ratio of formaldehyde and urea of 3:1. Pour all the formaldehyde 243.24g into the reaction kettle, add the first batch of urea 21.82g, adjust the pH value to 7.5, mix and stir, heat up to 95°C, react for 1h; adjust the pH value to 4.0 and continue the reaction for 1h; cool down to 85°C , add the second batch of urea 8.18g, adjust the pH to 7.0, and react for 1h; cool down to 75°C, add the third batch of urea 30g, react for 1h, and the reaction is completed to obtain UF resin.
[0029] Step 2, configure a nitric acid solution with a concentration of 7mol / L, and add NaNO to it 3 , Ba(NO 3 ) 2 , Sr(NO 3 ) 2 , CsNO 3 , so that the concentrations are 0.076 mol / L, 0.02 mol / L, 0.016 mol / L, 0.037 mol / L respectively, forming simulated high-level radioactive waste liquid.
[0030] Step 3, feed ammonia gas and add ammonium chloride to the good simulated high-level radioactive waste liquid configured in step...
Embodiment 2
[0031] Example 2: In Example 1, the concentration of hydrochloric acid in the reaction system was 0.1mol / L, and other conditions remained unchanged, and the removal rate of nitrate reached 27.25%.
Embodiment 3
[0032] Example 3: In Example 1, the concentration of hydrochloric acid in the reaction system was 0.5mol / L, and other conditions remained unchanged, and the removal rate of nitrate reached 39.74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com