Tricyclic diterpenoid derivative and preparation method as well as application of tricyclic diterpenoid derivative in preparation of neuroprotective drugs
A tricyclic diterpene and neuroprotective technology, which is applied in the field of medicine and its preparation and application, can solve the problems of limited compound resources and restricted development, and achieve the effect of simple synthesis method, environmental friendliness and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
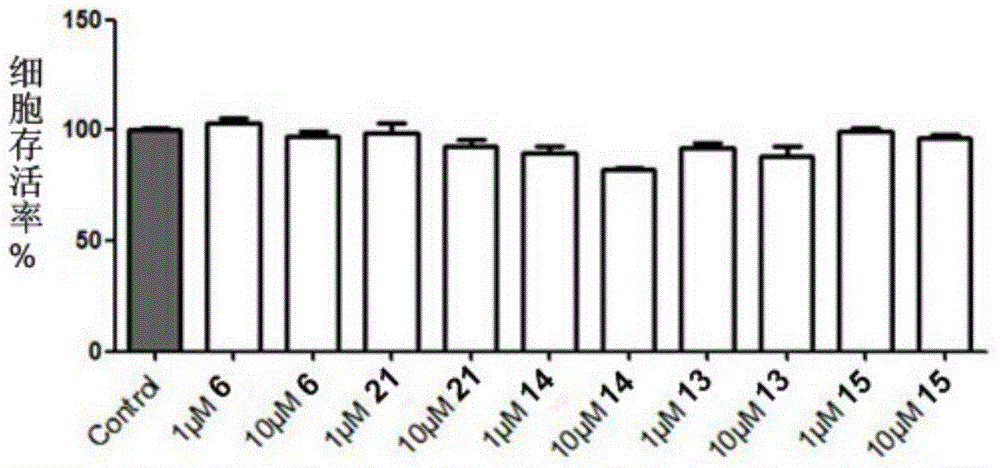
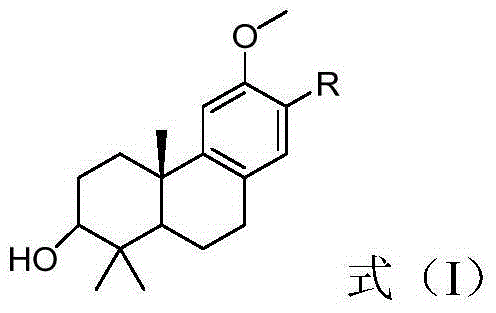
Image
Examples
Embodiment 1
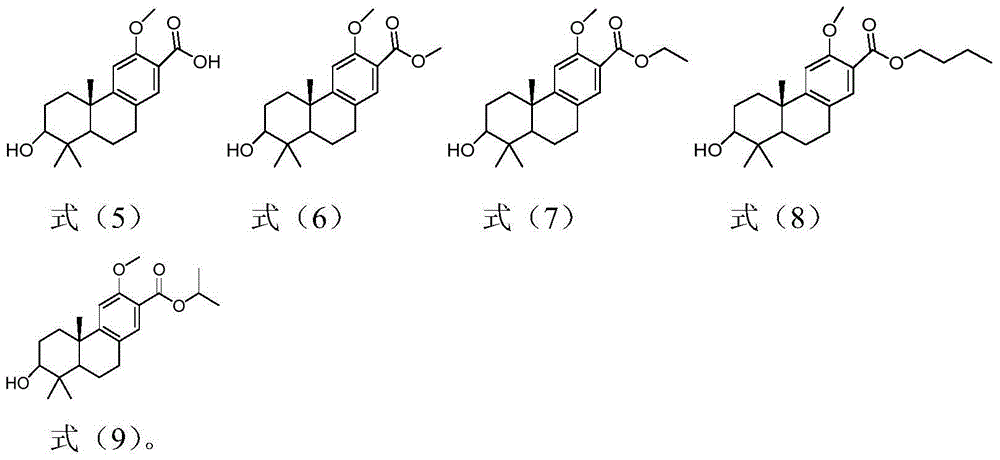
[0037] Embodiment 1: the preparation of tricyclic diterpene derivative (leader) shown in formula (5)
[0038]
[0039] The preparation of tricyclic diterpene derivatives shown in formula (5), that is, compound 5: Dissolve compound 1 (8g, 29, 19mmol) in 50mlDCM, slowly add Br 2 (1.5ml, 29.19mmol) of DCM solution 50ml, after the dropwise addition, stir for 1h, TLC detects that the reaction of the raw materials is complete, add water, extract the aqueous phase with DCM (30ml×3), combine the organic phases, wash with water (30ml×2), saturated NaHCO3 Wash (30ml×2), wash with saturated NaCl (30ml×2), dry over anhydrous sodium sulfate, and concentrate to obtain compound 2 (yellow oil), which is directly used in the next step.
[0040] The previous step crude product compound 2 (10.2g, 29mmol), TBSCl (6.5g, 43.5mmol), imidazole (3.95g, 58mmol) were placed in a single-necked bottle, injected into DMF50ml, N 2 Replace and stir overnight at room temperature. TLC detects that the rea...
Embodiment 2
[0043] Embodiment 2: the preparation of tricyclic diterpene derivatives shown in formula (6), (7), (8), (9)
[0044]
[0045] The preparation of tricyclic diterpene derivatives shown in formula (6), namely compound 6: compound 5 (60mg, 0.19mmol) was placed in a single-necked bottle, N 2 For replacement, inject 10ml of methanol, add 5 drops of concentrated sulfuric acid dropwise, and stir at room temperature. TLC detected that the reaction of the raw materials was complete, added 10ml of water, extracted the aqueous phase with EA (10ml×3), combined the organic phases, washed with water (10ml×2), washed with saturated NaCl (10ml×2), dried over anhydrous sodium sulfate, concentrated, and silica gel Column chromatography (PE:EA=2:1) and concentration gave product 6 (58 mg white solid, 91.8%). 1 H NMR (400MHz, CDCl 3 )δ7.49(s, 1H), 6.82(s, 1H), 3.85(s, 6H), 3.30(dd, J=11.1, 5.0Hz, 1H), 2.91(dd, J=16.8, 5.9Hz, 1H ), 2.82-2.71(m, 1H), 2.28(dt, J=12.9, 3.4Hz, 1H), 1.63-1.47(m,...
Embodiment 3
[0049] Embodiment 3: Preparation of tricyclic diterpene derivatives shown in formula (10), (11), (12), (13), (14), (15), (16), (17), (18)
[0050]
[0051] The preparation of tricyclic diterpene derivatives represented by formula (10), that is, compound 10: compound 5 (100 mg, 0.31 mmol), EDC.HCl (126 mg, 0.64 mmol), HOBt (86 mg, 0.64 mmol), DMAP (227 mg, 1.86mmol) and methylamine hydrochloride (64mg, 0.94mmol) were placed in a single-necked bottle, N 2 Replacement, inject 10ml of anhydrous DCM, stir overnight at room temperature. TLC detects that the reaction of the raw materials is complete, add 10ml of water, adjust the pH of the system to 1 H NMR (300MHz, CDCl 3 )δ7.89(s, 1H), 7.81(br.s, 1H), 6.80(s, 1H), 3.91(s, 3H), 3.31(dd, J=10.5, 5.5Hz, 1H), 3.10-2.60 (m, 5H), 2.28 (d, J=12.6Hz, 1H), 1.96-1.69 (m, 4H), 1.20 (s, 3H), 1.08 (s, 3H), 0.90 (s, 3H).
[0052] Preparation of tricyclic diterpene derivatives represented by formula (11), namely compound 11: Compound 5 (100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com