Method for synthesizing 2,3-dichloro-5-trichloromethyl pyridine
A technology of trichloromethylpyridine and chloromethylpyridine, applied in the field of halogenation reaction, can solve problems such as lack of yield, and achieve the effects of easy operation, avoidance of waste, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
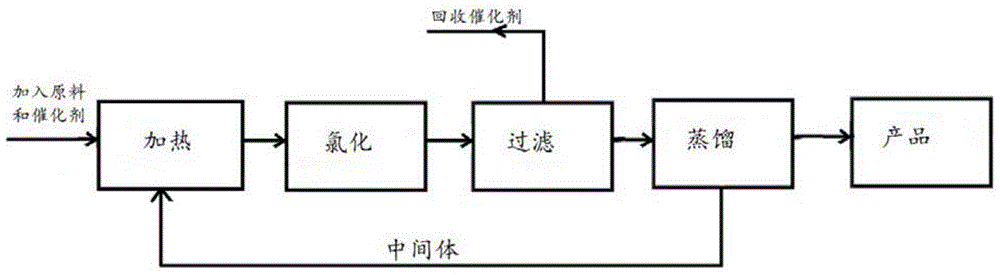
Image
Examples
Embodiment 1
[0029] During the experiment, the reaction temperature is explored, and the screening is usually carried out by continuously increasing the range of 10-15°C.
[0030] Under the condition of 1 atmospheric pressure, after heating 20 grams of 2-chloro-5-chloromethylpyridine (0.12mol) and 0.5 gram of manganese dioxide and 0.5 gram of ferric trichloride to 120 ° C, the mixture is fed with chlorine gas The time is 45h. Using GC-MS tracking detection, it was found that 36% of the raw material was converted into 2,3-dichloro-5-trichloromethylpyridine. When the temperature of the reaction system was heated to 170° C., chlorine gas was continued to be introduced for 7 h, and GC-MS tracking detection revealed that the raw materials had reacted completely. The reaction mixture was diluted with n-hexane, washed with aqueous sodium carbonate, the organic layer was separated, dried over anhydrous sodium sulfate and the solvent was removed by distillation to obtain 25.4 g of yellow liquid 2,...
Embodiment 2
[0032] The difference with Example 1 is: under the condition of 10 atmospheric pressure, to 2-chloro-5-chloromethylpyridine 162 grams (1mol) and ruthenium trichloride 4 grams (2.5W.t%), 4 grams MoCl O (2.5 W.t%) slowly feed 473 grams of chlorine into the mixture, all of which are heated to 175°C to 180°C, after 25h, the reaction mixture is cooled, and diluted with carbon tetrachloride to separate the organic phase, washed with sodium carbonate solution, and washed with anhydrous Na2SO4 dried. The solvent was evaporated to obtain 223 g of yellow oily liquid 2,3-dichloro-5-trichloromethylpyridine with a yield of 84%.
Embodiment 3
[0034] The difference with Example 1 is: under the condition of 6 atmospheric pressure, react under the condition of 170 ℃ with 40 grams of 2-chloro-5-chloromethylpyridine and 2.0 grams (5W.t%) catalyst cobalt dichloride After 16 hours, the product was post-treated to obtain 50 g of yellow liquid 2,3-dichloro-5-trichloromethylpyridine with a yield of 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com