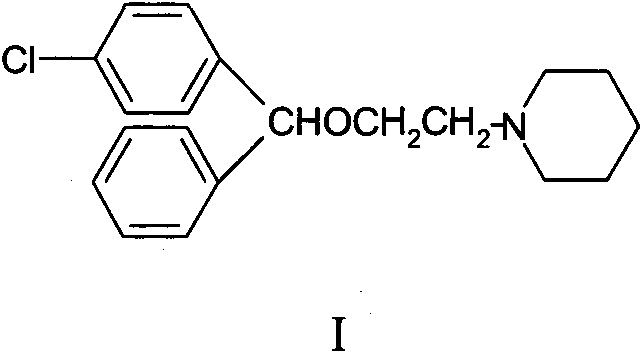
Industrial preparation method of cloperastine
A technology for cloperastine and piperidine, which is applied in the field of preparing cloperastine, can solve the problems of unsuitable industrialized production, high equipment corrosion, long reaction time, etc., and achieves low equipment corrosion, less pollution of three wastes, and catalytic effect. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
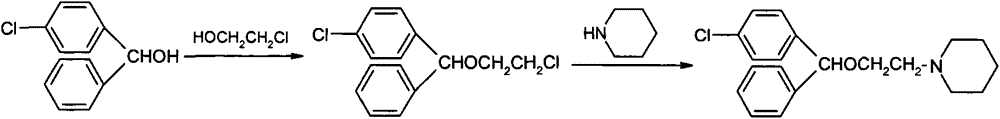
[0036] 1.1 Preparation of ether compound (III)
[0037] In the reactor, add 4-chlorobenzhydryl alcohol (V) (60.0Kg, 274.35mol), 2-chloroethanol (IV) (33.1Kg, 411.18mol), sodium bisulfate (10.8Kg, 90mol), toluene respectively 90.0Kg, start stirring, heat up to 95°C-100°C and keep it warm for 2 hours, cool to 45°C-50°C after the reaction, filter, wash the organic phase twice with 100Kg of drinking water, and concentrate the organic phase to dryness under reduced pressure to obtain Ether compound (III) (HPLC purity>99.0%, yield 94.5%).
[0038] 1.2 Preparation of cloperastine (I)
[0039] In the reaction kettle, add ether compound (III) (70.0Kg, 248.93mol), piperidine (II) (31.8Kg, 373.40mol), potassium carbonate (51.6Kg, 373.40mol), start stirring, and heat up to 100°C~ 105 ° C heat preservation reaction for 4 hours, cooled to 45 ° C ~ 50 ° C after the end of the reaction, filtered, added 300Kg purified water to the organic phase, precipitated crystals, recrystallized from abs...
Embodiment 2
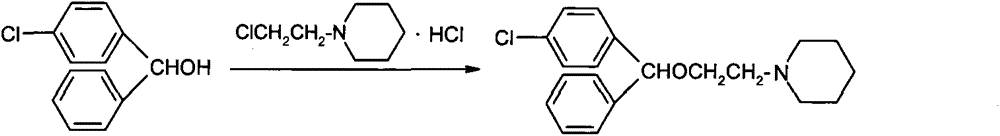
[0041] 2.1 Preparation of ether compound (III)
[0042] In the reactor, add 4-chlorobenzhydryl alcohol (V) (60.0Kg, 274.35mol), 2-chloroethanol (IV) (33.1Kg, 411.18mol), sodium bisulfate monohydrate (12.4Kg, 90mol) respectively , Toluene 90.0Kg, start stirring, heat up to 95°C-100°C and keep it warm for 2 hours, cool to 45°C-50°C after the reaction, filter, wash the organic phase twice with 100Kg of drinking water, and concentrate the organic phase to dryness under reduced pressure , to obtain ether compound (III) (HPLC purity>99.0%, yield 94%).
[0043] 2.2 Preparation of Cloperastine (I)
[0044]In the reaction kettle, add ether compound (III) (70.0Kg, 248.93mol), piperidine (II) (31.8Kg, 373.40mol), sodium carbonate (39.6Kg, 373.40mol), start stirring, and heat up to 100°C~ 105 ° C heat preservation reaction for 4 hours, cooled to 45 ° C ~ 50 ° C after the end of the reaction, filtered, added 300Kg purified water to the organic phase, precipitated crystals, recrystallized...
Embodiment 3
[0046] 3.1 Preparation of ether compound (III)
[0047] In the reactor, add 4-chlorobenzhydryl alcohol (V) (60.0Kg, 274.35mol), 2-chloroethanol (IV) (33.1Kg, 411.18mol), sodium bisulfate (10.8Kg, 90mol), toluene respectively 90.0Kg, start stirring, raise the temperature to 80°C-85°C and keep it warm for 2 hours, cool to 45°C-50°C after the reaction, filter, wash the organic phase twice with 100Kg drinking water, and concentrate the organic phase to dryness under reduced pressure to obtain Ether compound (III) (HPLC purity>99.0%, yield 89%).
[0048] 3.2 Preparation of Cloperastine (I)
[0049] In the reaction kettle, add ether compound (III) (68.0Kg, 241.82mol), piperidine (II) (30.9Kg, 362.73mol), potassium carbonate (33.4Kg, 241.82mol), start stirring, and heat up to 100°C~ 105 ° C heat preservation reaction for 4 hours, cooled to 45 ° C ~ 50 ° C after the end of the reaction, filtered, added 300Kg purified water to the organic phase, precipitated crystals, recrystallized ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com