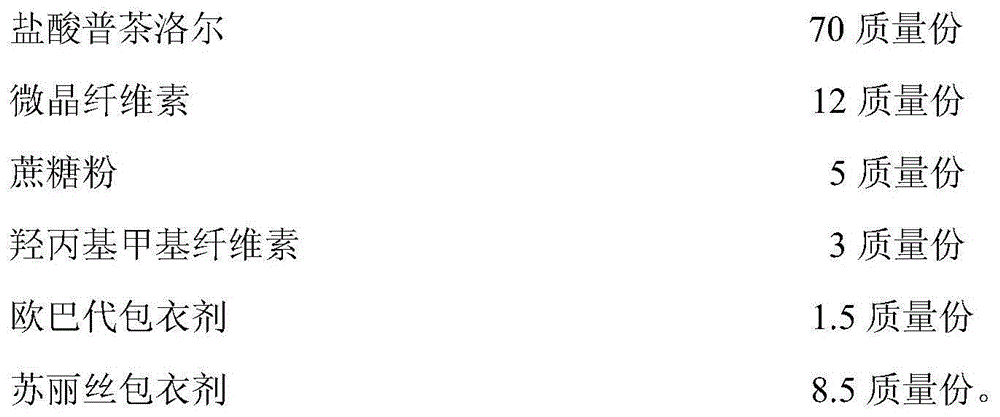Method for preparing cardiovascular drug sustained-release capsules
A sustained-release capsule and cardiovascular technology, which is applied to cardiovascular system diseases, drug delivery, drug combination, etc., to achieve the effects of reducing the number of medications, long action time, and large surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The composition prescription of capsule content described in the present embodiment is as follows:
[0033]
[0034] The preparation process of the sustained-release capsule described in the present embodiment is as follows:
[0035] a) First pass labetalol hydrochloride and filler microcrystalline cellulose through a 40-mesh sieve for pretreatment, then accurately weigh according to the prescription amount and mix well; then the binder hydroxypropyl methylcellulose (HPMC) Prepare a 2% aqueous solution and add it to the mixed material to make a soft material;
[0036] b) Extrude the prepared soft material into a short column with a 0.8mm screen, then use a centrifugal granulator to make pellets, and then dry them in a fluidized bed at 60±30°C;
[0037] c) sieve the dried pellets, and take 16-40 mesh pellets for later use; dissolve the remaining pellets with water into a solution or suspension, and then prepare 24-35 mesh pellets in a fluidized bed; combine the obtai...
Embodiment 2
[0040] The composition prescription of capsule content described in the present embodiment is as follows:
[0041]
[0042] The preparation process of the sustained-release capsule described in the present embodiment is as follows:
[0043] a) First pretreat pucharolol hydrochloride, filler microcrystalline cellulose and sucrose powder through a 40-mesh sieve, then accurately weigh and mix uniformly according to the prescription amount; then add the binder hydroxypropyl methylcellulose (HPMC) is formulated into a 0.5% aqueous solution, and added to the mixed material to make a soft material;
[0044] b) Extrude the prepared soft material into a short column with a 0.8mm screen, then use a centrifugal granulator to make pellets, and then dry them in a fluidized bed at 60±30°C;
[0045] c) sieve the dried pellets, and take 16-40 mesh pellets for later use; dissolve the remaining pellets with water into a solution or suspension, and then prepare 24-35 mesh pellets in a fluidi...
Embodiment 3
[0048] The composition prescription of capsule content described in the present embodiment is as follows:
[0049]
[0050]
[0051] The preparation process of the sustained-release capsule described in the present embodiment is as follows:
[0052] a) Firstly pass nifedipine and filler microcrystalline cellulose through a 60-mesh sieve for pretreatment, then accurately weigh according to the prescription amount and mix them evenly; then prepare the binder hydroxypropyl cellulose (HPC) to 1% aqueous solution, and add the mixed material to make soft material;
[0053] b) Extrude the prepared soft material into a short column with a 0.5mm screen, then use a centrifugal granulator to make pellets, and then dry them in a fluidized bed at 60±30°C;
[0054] c) Sieve the dried pellets, and take 20-50 mesh pellets for later use; dissolve the remaining pellets with water into a solution or suspension, and then prepare 16-40 mesh pellets in a fluidized bed; combine the obtained Al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com