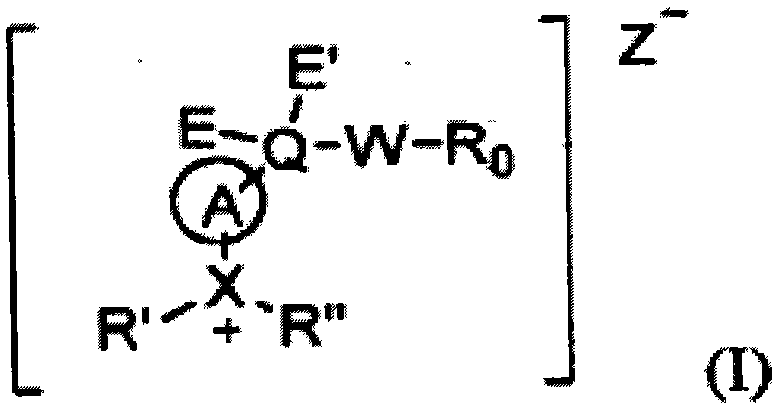
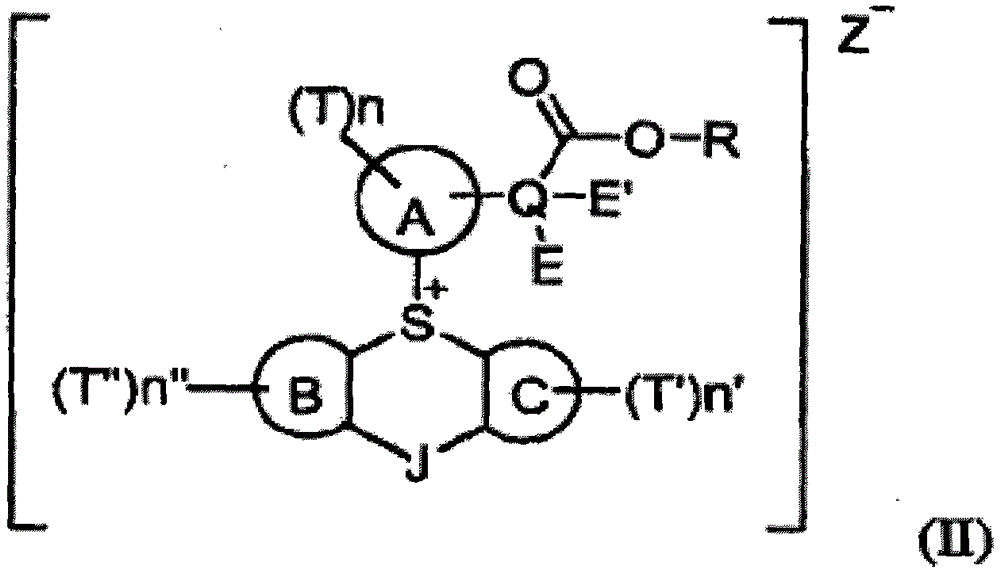
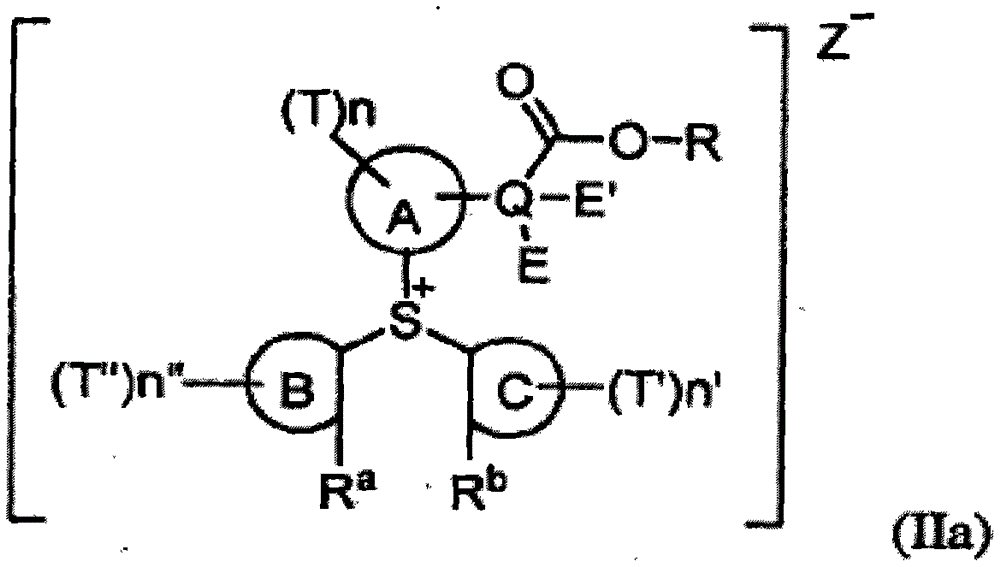
Substituted aryl onium materials
A substituent, heteroaryl technology, applied in the field of new onium acid generators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162]
[0163] Bis(4-(2-carboxypropan-2-yl)phenyl)iodonium bromide
[0164] 2-Methyl-2-phenylpropanoic acid (10 g, 60.9 mmol) was suspended in a 1:1 mixture of sulfuric acid and acetic acid (90 mL), and heated to 55 °C, where it was slowly dissolved in batches over 90 min. Join NaIO 4 (23.4 mmol, 5.00 g), followed by heating for an additional 1 hour. The reaction mixture was cooled, poured into ice water (1 L) and extracted with methyl tert-butyl ether (MTBE) (3 x 350 mL). Potassium bromide (0.609 mol, 72.4 g) in water (100 mL) was added to the aqueous layer and extracted with dichloromethane (DCM) (3 x 300 mL). The combined organic layers were concentrated and the crude residue was recrystallized from heptane, ethyl acetate and acetone to afford the title compound (5.27, 42%) as a white solid. 1 H NMR (300MHz, (CD 3 ) 2 SO) δ: 12.6 (brs, 2COOH), 8.16 (d, J = 8.1 Hz, 4H), 7.45 (d, J = 8.1 Hz, 4H), 1.43 (2, 12H).
Embodiment 2
[0166]
[0167] Bis(4-(2-carboxypropan-2-yl)phenyl)iodonium 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate
[0168] Bis(4-(2-carboxypropan-2-yl)phenyl)iodonium bromide (1.53 g, 2.87 mmol) and 1,1,2,2,3,3,4,4,4-nonafluoro Potassium butane-1-sulfonate (3.16 mmol, 1.07 g) was dissolved in DCM (50 mL) and water (50 mL) and stirred overnight. The layers were separated, the aqueous phase was extracted with DCM (2 x 100 mL), the combined organic phases were washed with water (2 x 100 mL) and concentrated to give the title compound (1.25 g, 58%) as a white solid. 1 H NMR (300MHz, (CD3)2CO) δ: 11.4 (brs, 2COOH), 8.34 (d, J = 7.8Hz, 4H), 7.64 (d, J = 7.8Hz, 4H), 1.57 (s, 12H).
Embodiment 3
[0170]
[0171] 5-(3-(Carboxymethyl)-4-methoxyphenyl)-dibenzothiophene iodide
[0172] Eaton's reagent (phosphorus pentoxide solution in methanesulfonic acid) (400 mL) was added to 2-methoxyphenylacetic acid (140 g, 0.843 mol) and dibenzothiophene oxide (160 g, 0.800 mol) A solution in dichloromethane (400 mL) was stirred at 25°C for 18 hours, cooled to 0°C, and carefully quenched with water (1 L). The aqueous mixture was washed with MTBE (3 x 500 mL) and the aqueous phase was poured into aqueous sodium iodide (300 g in 3 L) followed by vigorous stirring for 1 h. The precipitate was filtered, washed with water (3 x 1 L), acetone (1 L) and MTBE (2 x 500 mL) to afford the title compound as a white solid (360 g, 94%). 1 HNMR (500MHz, (CD3) 2 SO) δ: 12.30(brs, COOH), 8.52(d, J=7.5Hz, 2H), 8.31(d, J=8.5Hz, 2H), 7.95(t, J=7Hz, 2H), 7.75(t, J=8Hz, 2h), 7.70(dd, J=9, 2Hz, 1H), 7.31(d, J=2Hz, 1H), 7.23(d, J=8.5Hz, 1H), 3.82(s, 3H), 3.46(s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com