Hot-melt extrusion preparation of pazopanib hydrochloride and preparation method of hot-melt extrusion preparation
A technology for pazopanib hydrochloride and solid pazopanib hydrochloride is applied in the field of hot-melt extrusion preparation of pazopanib hydrochloride and its preparation, which can solve the problems of limiting wide application, thermal decomposition of drugs and carriers, etc. The preparation process is simple, the dosage is reduced, and the effect of no solvent residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
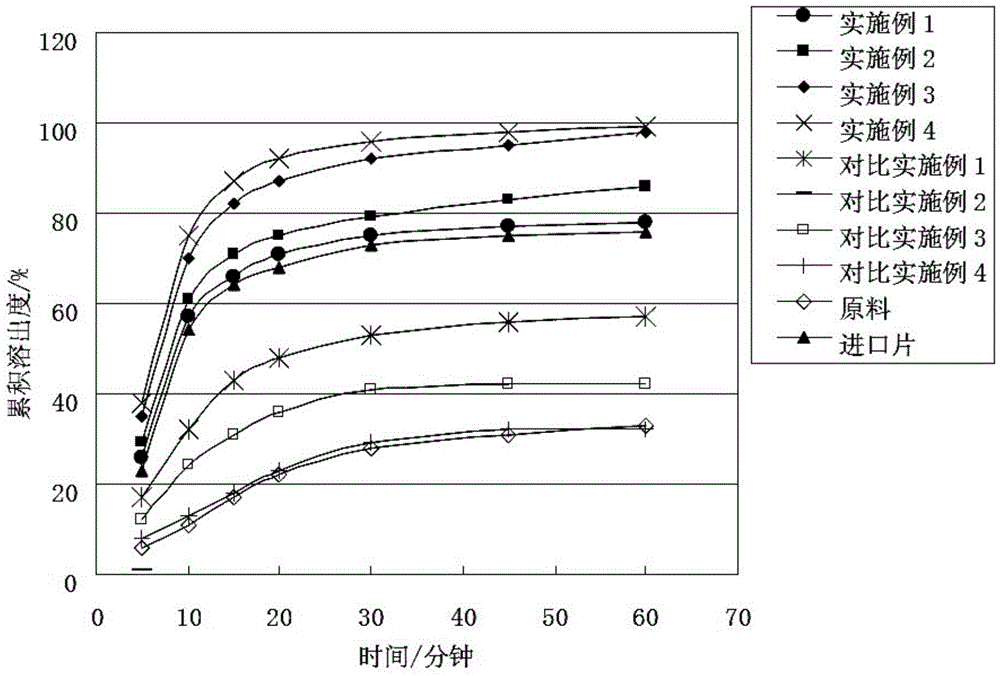
Examples
Embodiment 1
[0044] Pazopanib hydrochloride 1 part
[0045] PVP-S630 12 copies
[0046] Macrogol 4000 2 parts
[0047] Preparation process: mix uniformly to prepare a physical mixture, set the temperature of the extruder at 180°C, after the temperature rises to the set value and stabilize, add the physical mixture at a uniform speed, melt, extrude to obtain strips, cool, and crush for 40 mesh sieve to obtain pazopanib hydrochloride solid dispersion particles.
Embodiment 2
[0049] Pazopanib hydrochloride 1 part
[0050] PVP-VA64 7 copies
[0051] Macrogol 4000 2 parts
[0052] Preparation process: mix uniformly to prepare a physical mixture, set the temperature of the extruder at 150°C, after the temperature rises to the set value and stabilize, add the physical mixture at a uniform speed, melt, extrude to obtain strips, cool, and crush for 40 mesh sieve to obtain pazopanib hydrochloride solid dispersion particles.
Embodiment 3
[0054] Pazopanib hydrochloride 1 part
[0055] PVP-VA64 10 copies
[0056] Macrogol 4000 4 parts
[0057] Preparation process: mix evenly to prepare a physical mixture, set the temperature of the extruder at 165°C, after the temperature rises to the set value and stabilize, add the physical mixture at a uniform speed, melt, extrude to obtain strips, cool, and crush for 40 mesh sieve to obtain pazopanib hydrochloride solid dispersion particles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com