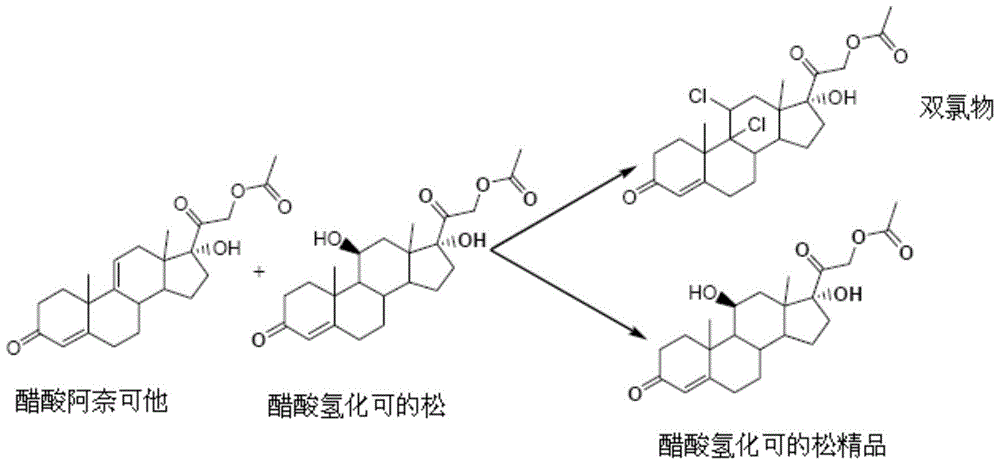
Refining method of hydrocortisone acetate
A technology of hydrocortisone acetate and a refining method, which is applied in organic chemistry, steroid compounds, etc., can solve the problems of cumbersome operation procedures, high production costs, and large environmental pollution, and achieve stable process, simple operation, and simple reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A method for refining hydrocortisone acetate of the present invention, comprising the following steps: adding 1 weight part of hydrocortisone acetate crude product, 6 parts by weight of methylene chloride, and 5 parts by weight of methanol to a reactor, heating and refluxing to dissolve Clear, filter, and keep the filter system at 30°C, and feed 0.1 parts by weight of chlorine gas. After the addition, stir for half an hour, concentrate to remove dichloromethane, stop the concentration when the residual amount of methanol reaches 1 part by weight, cool down to 0°C, and centrifuge to obtain the refined product of hydrocortisone acetate. Refined yield: 80.5%, HPLC=99.20%, external standard content: 97.10%, conforming to EP 8.0. The HPLC retention time is consistent with the standard control.
Embodiment 2
[0023] A method for refining hydrocortisone acetate of the present invention, comprising the following steps: adding 1 weight part of hydrocortisone acetate crude product, 8 weight parts of dichloromethane, and 6 weight parts of methanol to a reactor, and heating up to reflux until dissolved Clear, filter, and keep the filtration system at 40°C, then add 0.5 parts by weight of sodium hypochlorite, 0.1 parts by weight of sodium chloride, and then dropwise add glacial acetic acid, the total amount of glacial acetic acid is 0.35 parts by weight. Chloromethane, stop concentrating when the residual amount of methanol reaches 1 weight, cool down to 5°C, and centrifuge to obtain hydrocortisone acetate fine product. Refined yield: 81.5%, HPLC=99.10%, external standard content: 98.10%, conforming to EP 8.0. The HPLC retention time is consistent with the standard control.
Embodiment 3
[0025] A method for refining hydrocortisone acetate of the present invention, comprising the steps of: adding 1 weight part of hydrocortisone acetate crude product, 10 weight parts of dichloromethane, and 4 weight parts of methanol to a reactor, and heating up to reflux until dissolved Clear, filter, keep the filtration system at 35°C, add 0.5 parts by weight of calcium hypochlorite, 0.1 parts by weight of sodium chloride, and then dropwise add glacial acetic acid, the total amount of glacial acetic acid is 0.25 parts by weight, after adding, stir for half an hour, concentrate The dichloromethane was removed, the concentration was stopped when the residual amount of methanol was 1.5 parts by weight, the temperature was lowered to 10° C., and the mixture was centrifuged to obtain the refined product of hydrocortisone acetate. Refined yield: 80.3%, HPLC=99.12%, external standard content: 97.30%, conforming to EP 8.0. The HPLC retention time is consistent with the standard contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com