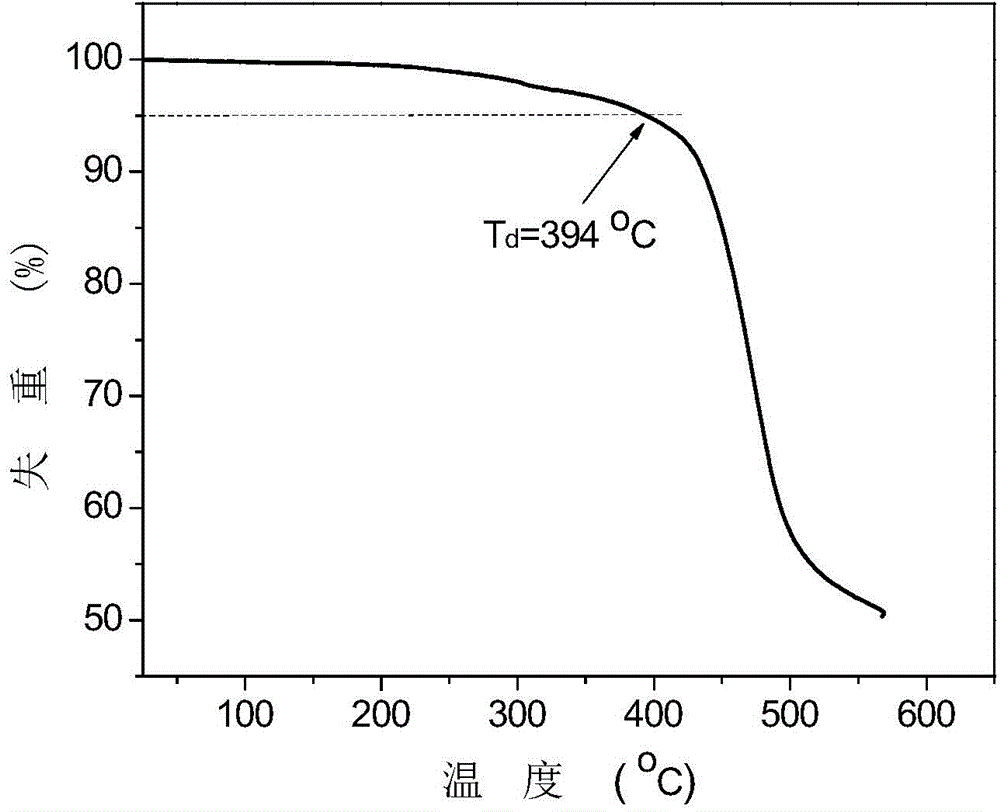
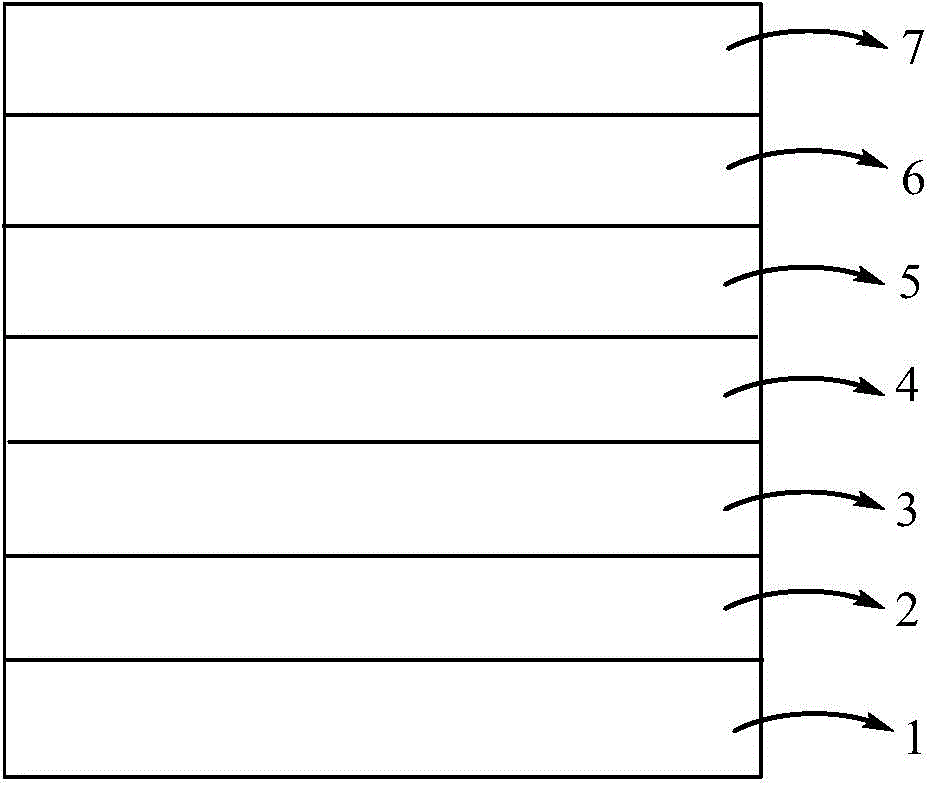
Bipolar blue-ray phosphor compound, as well as preparation method and organic electroluminescent device thereof
A compound and bipolar technology, applied in the field of organic electroluminescent materials, can solve the problems of high triplet energy level, lack of carrier transport performance, etc., achieve good thermal stability, excellent hole transport performance, and improve luminescence efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
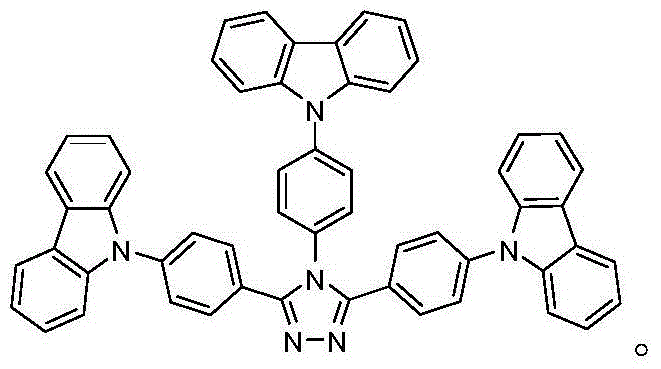
[0035] Example 1: The bipolar blue phosphorescent compound of this example is: 9-(4-(3,5-bis(4-(9H-carbazole)phenyl)-4H-1,2,4-tri Azol-4-yl)phenyl)-9H-carbazole, the structural formula is as follows:
[0036]
[0037] The preparation process of this compound is as follows:
[0038]
[0039] Under nitrogen protection, 9-(4-(3,5-bis(4-bromophenyl)-4H-1,2,4-triazol-4-yl)phenyl)-9H-carbazole (49.6 g, 80mmol) was dissolved in 200mL N,N-dimethylformamide (DMF) solution, then added 9H-carbazole (26.7g, 160mmol), potassium carbonate (22.1g, 160mmol), cuprous iodide (1.52g ,8mmol). The mixture was stirred and reacted at 120°C for 6 hours. The reaction was stopped and cooled to room temperature, filtered, and the solid was washed three times with distilled water. The crude product was separated by silica gel column chromatography using n-hexane as the eluent, and then dried under vacuum at 50°C for 24 hours to obtain a gray-white solid bipolar blue phosphorescent compound. The...
Embodiment 2
[0041] Example 2: The bipolar blue light phosphorescent compound of this example is: 9-(4-(3,5-bis(4-(9H-carbazole)phenyl)-4H-1,2,4-tri Azol-4-yl)phenyl)-9H-carbazole, the structural formula is as follows:
[0042]
[0043] The preparation process of this compound is as follows:
[0044]
[0045] Under nitrogen protection, 9-(4-(3,5-bis(4-bromophenyl)-4H-1,2,4-triazol-4-yl)phenyl)-9H-carbazole (49.6 g, 80mmol) was dissolved in 200mL toluene (Tol) solution, then 9H-carbazole (29.4g, 176mmol), cesium carbonate (57.2g, 176mmol), copper powder (0.768g, 12mmol) were added. The mixture was stirred and reacted at 110°C for 9 hours. The reaction was stopped and cooled to room temperature, filtered, and the solid was washed three times with distilled water. The crude product was separated by silica gel column chromatography using n-hexane as the eluent, and then dried under vacuum at 50°C for 24 hours to obtain a gray-white solid bipolar blue phosphorescent compound. The yield...
Embodiment 3
[0046]Example 3: The bipolar blue phosphorescent compound of this example is: 9-(4-(3,5-bis(4-(9H-carbazole)phenyl)-4H-1,2,4-tri Azol-4-yl)phenyl)-9H-carbazole, the structural formula is as follows:
[0047]
[0048] The preparation process of this compound is as follows:
[0049]
[0050] Under nitrogen protection, 9-(4-(3,5-bis(4-bromophenyl)-4H-1,2,4-triazol-4-yl)phenyl)-9H-carbazole (49.6 g, 80mmol) was dissolved in 200mL of acetonitrile (MeCN) solution, and then 9H-carbazole (32.0g, 192mmol), potassium phosphate (39g, 184mmol), cuprous oxide (2.3g, 16mmol) were added. The mixture was stirred and reacted at 90°C for 12 hours. The reaction was stopped and cooled to room temperature, filtered, and the solid was washed three times with distilled water. The crude product was separated by silica gel column chromatography using n-hexane as the eluent, and then dried under vacuum at 50°C for 24 hours to obtain a gray-white solid bipolar blue phosphorescent compound. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com