Bipolar blue-ray phosphorescent main body material as well as preparation method and application thereof
A phosphorescent main body and bipolar technology, which is applied in the direction of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve problems such as shortage, achieve the effects of reduced manufacturing costs, good thermal stability, and improved luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
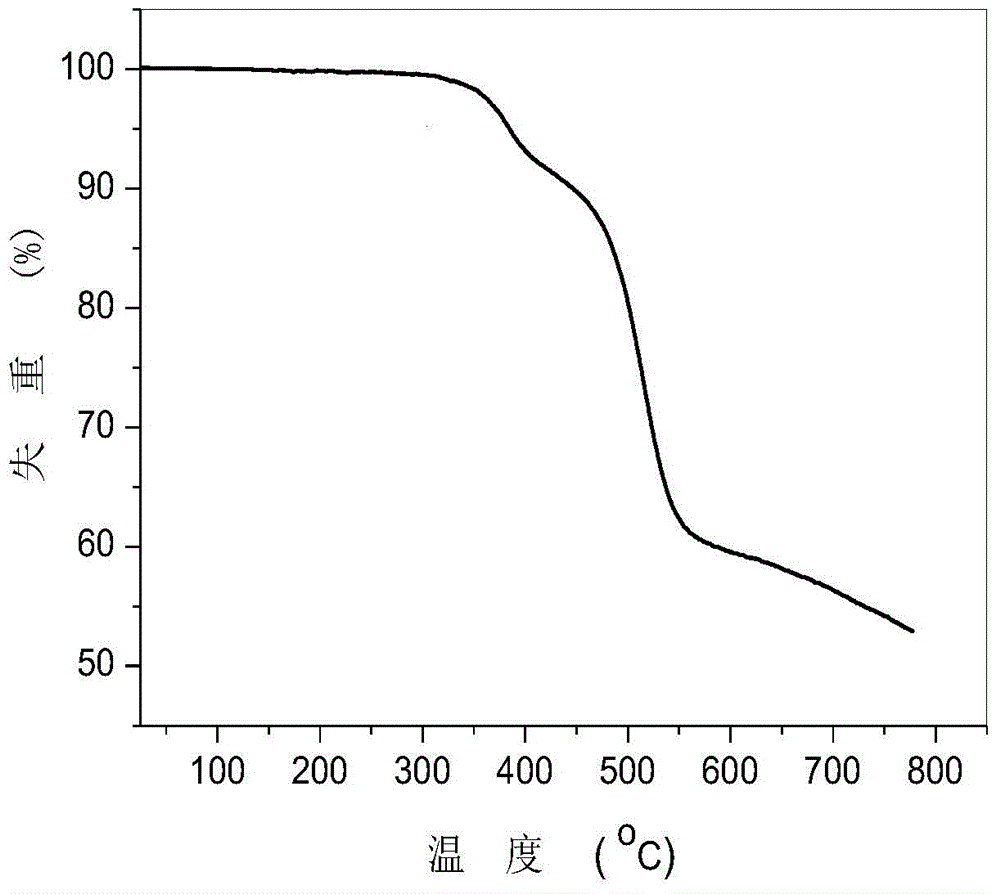
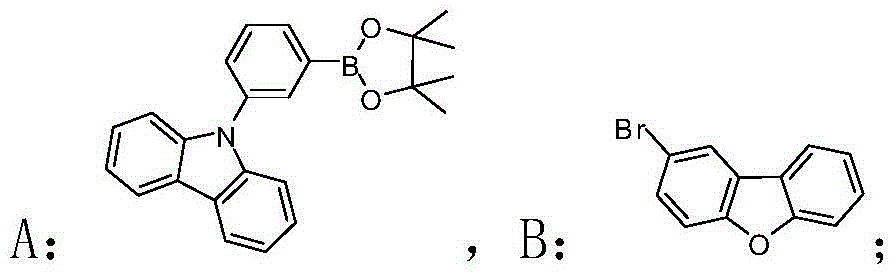
[0040] Example 1: The bipolar blue phosphorescence host material of this example is: 9-(3-(dibenzo[b,d]furan-2-yl)phenyl)-9H-carbazole, the preparation steps are as follows :
[0041]
[0042] The preparation steps of above-mentioned small organic molecules are as follows:
[0043] The reaction formula is as follows:
[0044]
[0045] Under argon protection, 9-(3-pinacol borate phenyl)-9H-carbazole (74mg, 0.2mmol), 2-bromodibenzo[b,d]furan (49mg, 0.2mmol) Add it to a flask containing 10ml of toluene solvent, and after fully dissolving, add a solution of potassium carbonate (2mL, 2mol / L) into the flask, vacuumize the oxygen and fill it with argon, then add bistriphenylphosphine palladium dichloride (5.6mg, 0.008mmol); the flask was heated to 120°C for Suzuki coupling reaction for 24h. Stop the reaction and cool to room temperature, extract three times with dichloromethane, then dry the organic phase with anhydrous magnesium sulfate and spin dry to obtain the crude prod...
Embodiment 2
[0047] Example 2: The bipolar blue phosphorescence host material of this example is: 9-(3-(dibenzo[b,d]furan-2-yl)phenyl)-9H-carbazole, the preparation steps are as follows :
[0048]
[0049] The preparation steps of above-mentioned small organic molecules are as follows:
[0050] The reaction formula is as follows:
[0051]
[0052] Under the protection of mixed gas of nitrogen and argon, 9-(3-pinacol borate phenyl)-9H-carbazole (111mg, 0.3mmol), 2-bromodibenzo[b,d]furan (81.2 mg, 0.33mmol) and 15mL tetrahydrofuran into a 50mL two-necked bottle, fully dissolved, and then a mixture of nitrogen and argon was introduced to exhaust the air for about 20 minutes, and then tetrakistriphenylphosphine palladium (4mg, 0.003mmol) was added to it , fully dissolved and then added sodium bicarbonate (3mL, 2mol / L) solution. After the mixed gas of nitrogen and argon was sufficiently exhausted for about 10 minutes, the two-neck flask was added to 70° C. for Suzuki coupling reaction fo...
Embodiment 3
[0053] Example 3: The bipolar blue phosphorescence host material of this example is: 9-(3-(dibenzo[b,d]furan-2-yl)phenyl)-9H-carbazole, the preparation steps are as follows :
[0054]
[0055] The preparation steps of above-mentioned small organic molecules are as follows:
[0056] The reaction formula is as follows:
[0057]
[0058] Under nitrogen protection, 9-(3-pinacol borate phenyl)-9H-carbazole (111mg, 0.3mmol), 2-bromodibenzo[b,d]furan (88.6mg, 0.36mmol), Palladium acetate (3.5mg, 0.015mmol) and tri(o-methylphenyl)phosphine (21mg, 0.06mmol) were added to a flask containing 12mL of N,N-dimethylformamide, and potassium carbonate was added after fully dissolving (3mL, 2mol / L) solution, and then pass nitrogen gas into the flask to evacuate the air for about 30min; heat the flask to 130°C for Suzuki coupling reaction for 12h. Stop the reaction and cool to room temperature, extract three times with dichloromethane, then dry the organic phase with anhydrous magnesium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com