Solid dispersion of cilnidipine and preparation method thereof
A solid dispersion and cilnidipine technology, which is applied in pill delivery, cardiovascular system diseases, powder delivery, etc., can solve the problem that cilnidipine dispersion is difficult to achieve disintegration and dissolution effects, and it is difficult to play a role in maintaining hypertension and other issues to achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
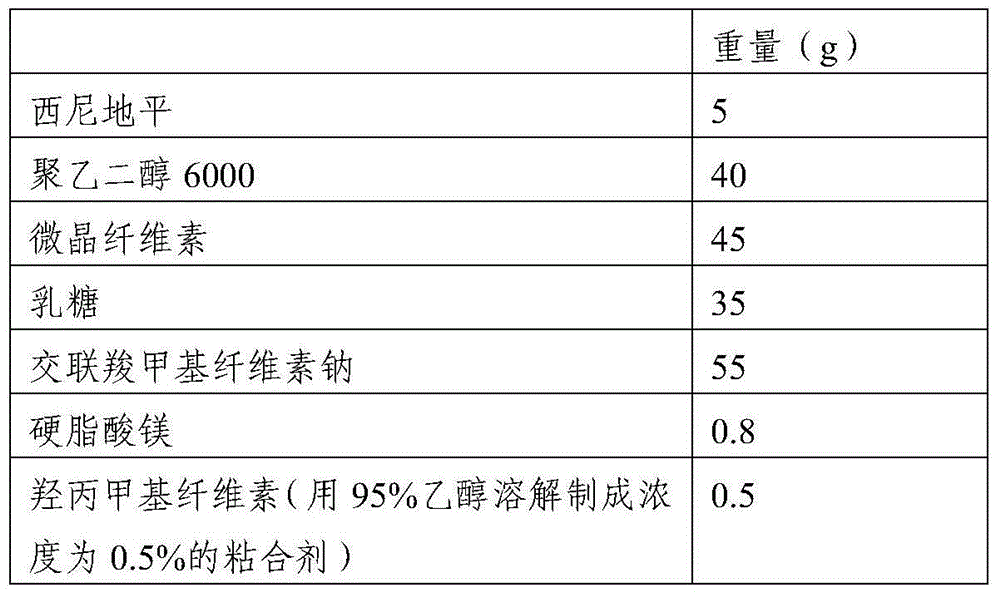
[0030] 1. Raw materials and prescription composition
[0031]
Weight (g)
cilnidipine
5
polyethylene glycol 6000
45
55
25
52.5
0.7
0.5% hypromellose (dissolved in 95% ethanol)
0.5
[0032] 2. Preparation method:
[0033] 1) Put polyethylene glycol 6000 in a water bath and heat it in a water bath to melt it. Control the temperature of the water bath at 75-80°C, and then add cilnidipine. The weight ratio of cilnidipine to polyethylene glycol 6000 is 1: 5. Stir fully to make it heated evenly and melt until the material melts into a yellow transparent solution and the bubbles completely disappear; the dispersion solution is obtained, and then the dispersion solution is flattened and frozen at a temperature of -13°C to -15°C. Freeze overnight, pulverize, and pass through an 80-mesh sieve to obtain the cilnidi...
Embodiment 2
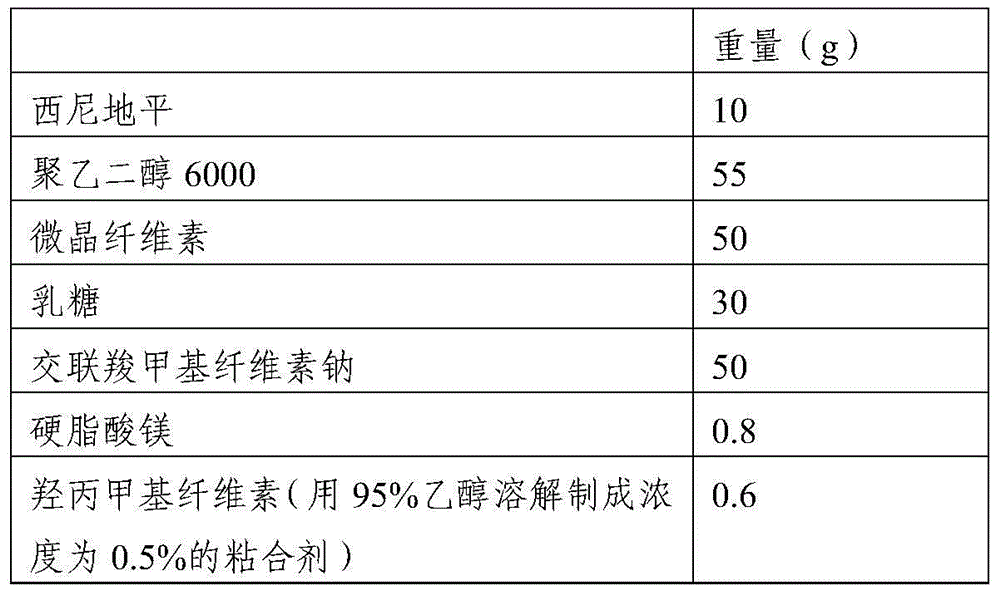
[0036] 1. Raw materials and prescription composition
[0037]
[0038] 2. Preparation method
[0039] 1) Put polyethylene glycol 6000 in a water bath and heat it in a water bath to melt it. Control the temperature of the water bath at 70-75°C, and then add cilnidipine. The weight ratio of cilnidipine to polyethylene glycol 6000 is 1: 10. Stir fully to make it evenly heated and melted until the material melts into a yellow transparent solution and the bubbles completely disappear; the dispersion solution is obtained, and then the dispersion solution is flattened and frozen at a temperature of -13°C to -15°C. Freeze overnight, pulverize, and pass through an 80-mesh sieve to obtain the cilnidipine dispersion powder;
[0040] 2) Weigh each component according to the formula, add auxiliary materials except binder and magnesium stearate to the cilnidipine dispersion powder, mix well, then add binder to granulate, dry at 45°C, granulate, add Magnesium stearate, direct compressio...
Embodiment 3
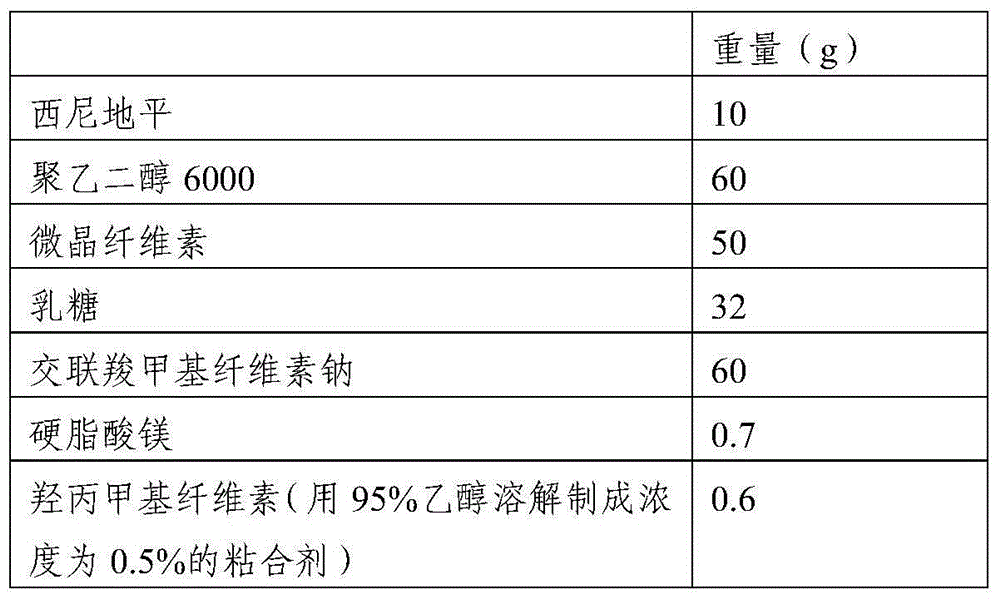
[0042] 1. Raw materials and prescription composition
[0043]
[0044] 2. Preparation method
[0045]1) Put polyethylene glycol 6000 in a water bath and heat it in a water bath to melt it. Control the temperature of the water bath at 70-75°C, and then add cilnidipine. The weight ratio of cilnidipine to polyethylene glycol 6000 is 1: 10. Stir fully to make it evenly heated and melted until the material melts into a yellow transparent solution and the bubbles completely disappear; the dispersion solution is obtained, and then the dispersion solution is flattened and frozen at a temperature of -13°C to -15°C. Freeze overnight, pulverize, and pass through an 80-mesh sieve to obtain the cilnidipine dispersion powder;
[0046] 2) Weigh each component according to the formula, add auxiliary materials except binder and magnesium stearate to the cilnidipine dispersion powder, mix well, then add binder to granulate, dry at 45°C, granulate, add Magnesium stearate, direct compression...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com