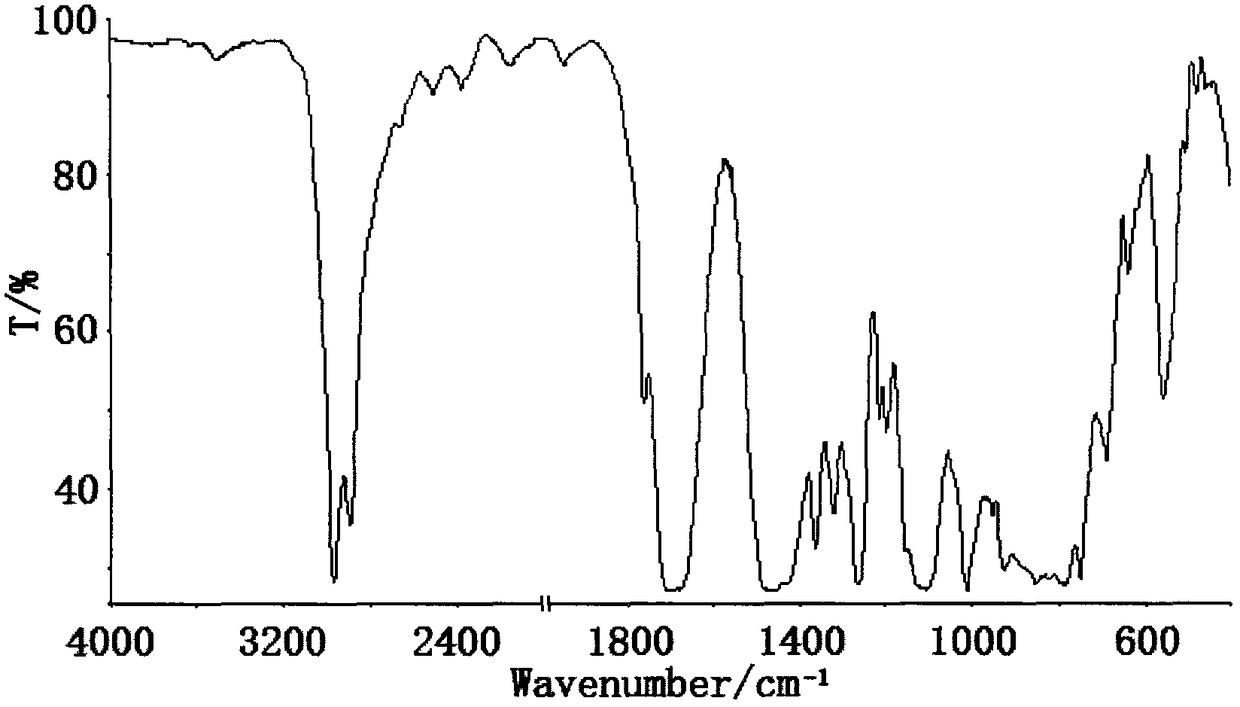
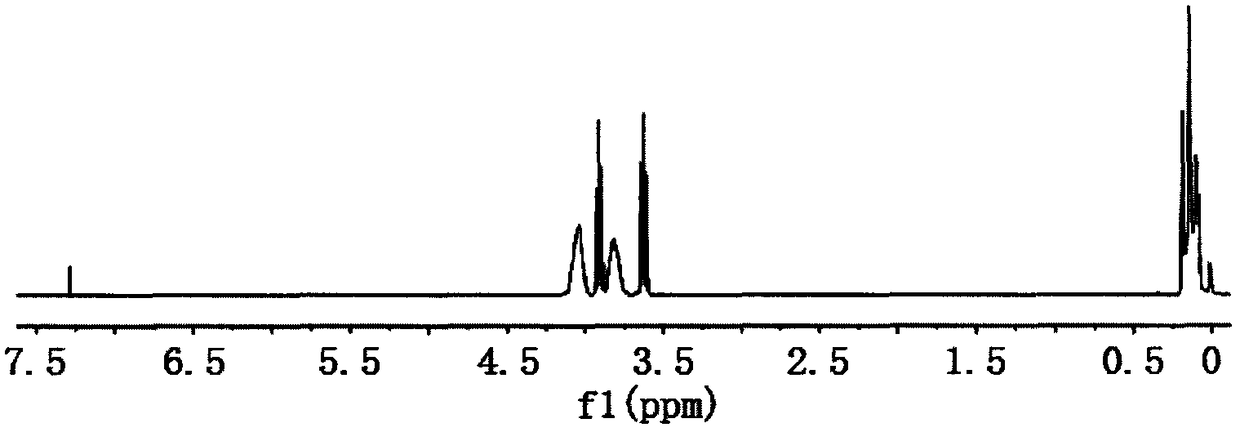
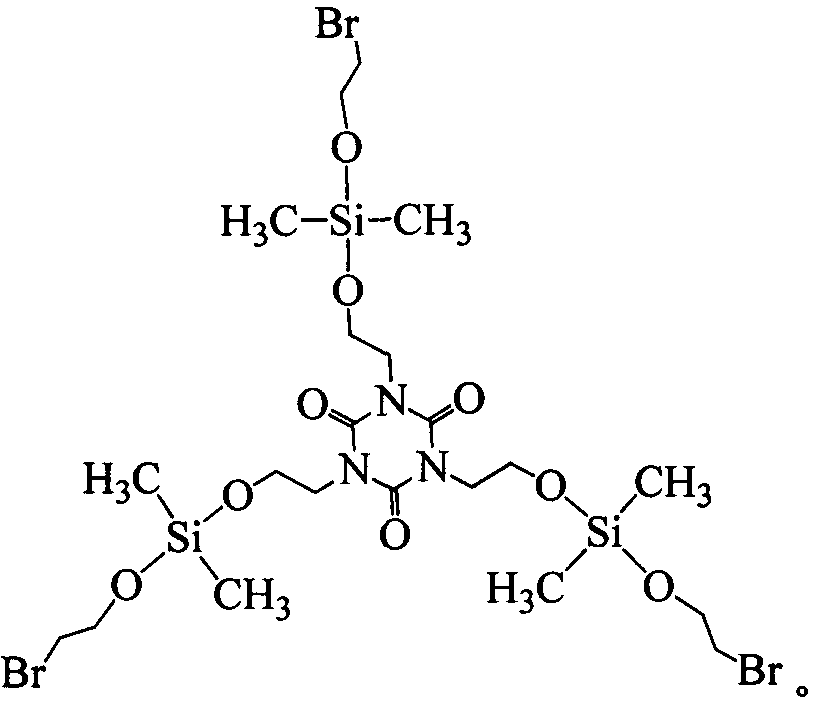
Tris(2-dimethylbromoethoxysilyloxyethyl) isocyanurate compound and preparation method thereof
A technology of dimethylbromoethoxysilyloxyethyl and isocyanuric acid three, which is applied in the field of flame retardant plasticizers, can solve environmental and human health hazards, restrictions on the use of halogenated flame retardants, The impact of mechanical processing performance is small, and it is beneficial to environmental protection, good economic and environmental benefits, and good compatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 In the 250ml four-necked bottle that stirrer, thermometer and condensation tube are equipped with, and drying tube and hydrogen bromide absorption device are equipped with at the top of condensation tube, replace the air in the bottle with nitrogen, add 8.74g ( 0.0333mol) tris (2-hydroxyethyl) isocyanurate and 80ml dioxane, add 21.8g (0.1mol) dimethyldibromosilane, heat up to 50°C, keep warm for 5h; Finally, lower the temperature to below 5°C, replace the drying tube with a highly expandable sealing sleeve, and introduce 4.41g (0.1mol) of ethylene oxide under the liquid surface, and control the reaction temperature not higher than 15°C at the rate of introduction. 2 hours after the completion of the passage, the temperature was raised to 40°C, and the temperature was kept for 4 hours; the solvent and a small amount of low boiling point were removed by distillation under reduced pressure to obtain tris(2-dimethylbromoethoxysilyloxyethyl) isocyanurate, which p...
Embodiment 2
[0028] Embodiment 2 In the 250ml four-necked bottle that stirrer, thermometer and condensation tube are equipped with, and drying tube and hydrogen bromide absorption device are equipped with at the top of condensation tube, replace the air in the bottle with nitrogen, add 8.74g ( 0.0333mol) tris (2-hydroxyethyl) isocyanurate and 100ml tetrahydrofuran, add 21.8g (0.1mol) dimethyldibromosilane, heat up to 30°C, keep warm for 9h; after the HBr is released, cool down When the temperature is lower than 5°C, replace the drying tube with a highly expandable sealing sleeve, and introduce 4.70g (0.1067mol) of ethylene oxide under the liquid surface, and control the reaction temperature at a rate not higher than 15°C. Reaction at 15°C for 8 hours; normal pressure distillation to remove excess ethylene oxide, and then vacuum distillation to remove solvent and a small amount of low boiling point substances to obtain tris(2-dimethylbromoethoxysilyloxyisocyanurate) Ethyl) ester, yield 93.7...
Embodiment 3
[0029] Embodiment 3 In the 250ml four-necked bottle that stirrer, thermometer and condensation tube are equipped with, and drying tube and hydrogen bromide absorption device are equipped with at the top of condensation tube, replace the air in the bottle with nitrogen, add 8.74g ( 0.0333mol) tris (2-hydroxyethyl) isocyanurate and 120ml acetonitrile, add 21.8g (0.1mol) dimethyldibromosilane, heat up to 45°C, keep warm for 6h; after the HBr is released, cool down When the temperature is lower than 5°C, replace the drying tube with a highly expandable sealing sleeve, and inject 5.14g (0.1167mol) of ethylene oxide under the liquid surface, and control the reaction temperature at the rate of introduction to not exceed 15°C. Heat up to 25°C for 1 hour, keep warm for 6 hours; remove excess ethylene oxide by distillation under normal pressure, and then remove solvent and a small amount of low boiling point by distillation under reduced pressure to obtain tris(2-dimethylbromoethoxysilan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com