Peptide, peptide derivatives, medicinal salts of peptide, medicine composition and application of peptide and peptide derivatives
A technology of peptide derivatives and compositions, applied in the direction of drug combinations, microorganisms, biochemical equipment and methods, etc., can solve the problems of short plasma half-life, achieve long plasma half-life, good stability, and improve the function of islets of pancreas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
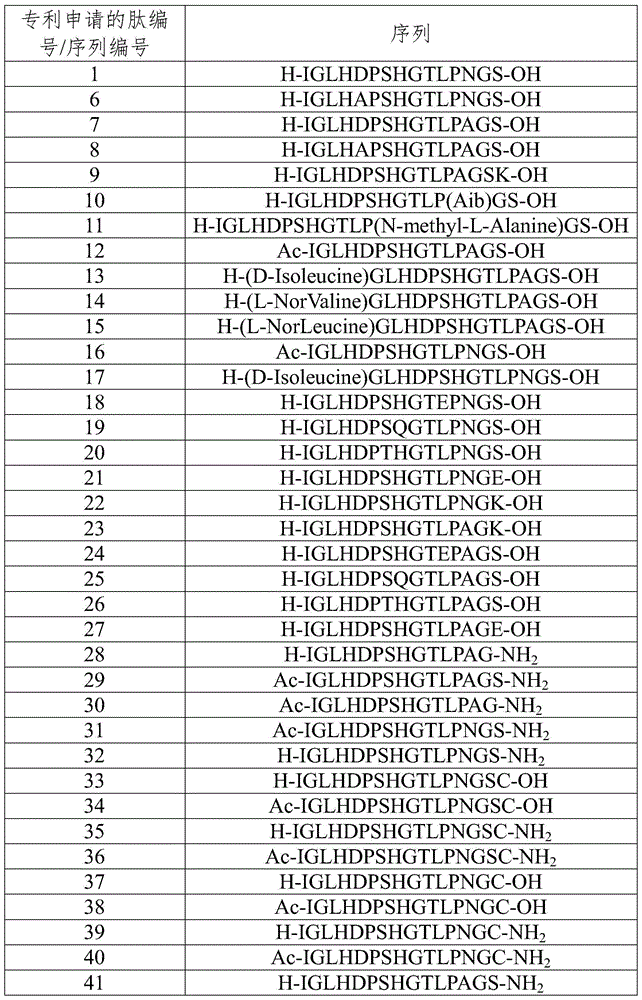
[0128] The production of embodiment 1 polypeptide
[0129] This example describes the production of polypeptides
[0130] All peptides used in this study were synthesized using 9-fluorenylmethyl chloroformate (Fmoc) solid-phase synthesis. Briefly, a weighed amount of 2-chlorotrityl chloride resin (1.6 mmol / g) was dissolved in dichloromethane (DCM). For C-terminally amidated peptides of interest, use Rink amide resin instead of 2-chlorotrityl chloride resin. For coupling reactions in the presence of hydroxybenzotriazole (Sigma Chemicals, Inc., St. Louis, MO, USA) in dimethylformamide (DMF), preactivated Fmoc-amino acids were used. The entire synthesis process uses an excess of amino acids. Deprotection of the Fmoc group in 20% piperidine in DMF leads to chain extension reactions. When the chain extension reaction was completed, the Fmoc protecting group was removed from the N-terminus of the polypeptide using DMF containing 25% piperidine, and then washed four times with DM...
Embodiment 2
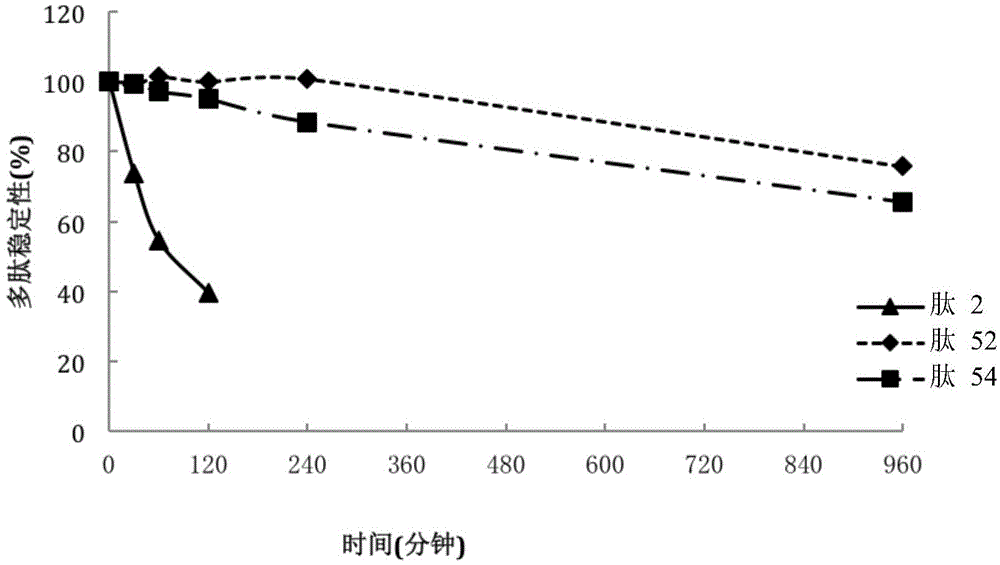
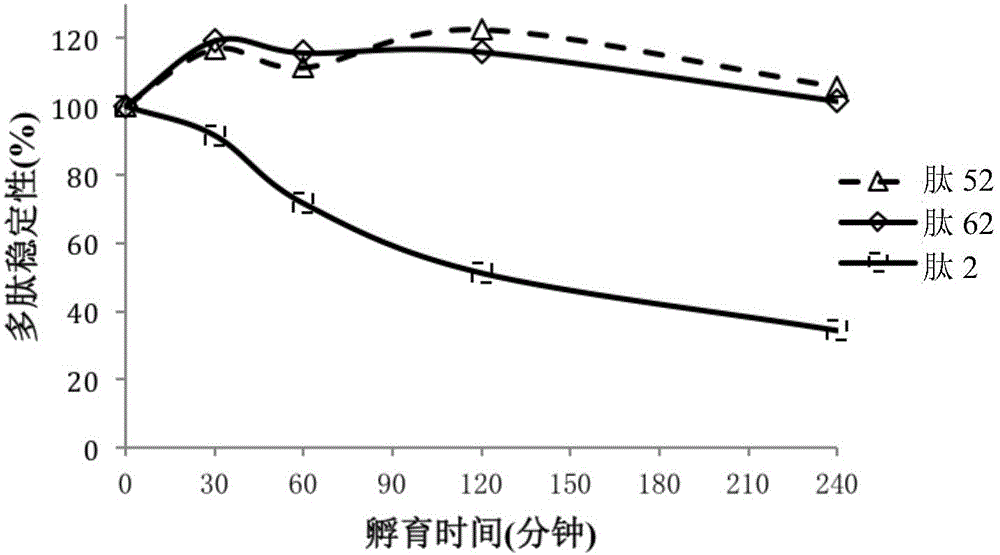
[0134] Stability experiment of embodiment 2 peptide
[0135] This example describes stability experiments of peptides under various conditions.
[0136] Test the stability of the peptide in mouse and rat plasma. A brief description is as follows: Accurately weigh a certain amount of polypeptide and eucatropine (positive control), dissolve the compound to be tested in 50% methanol-water solution, and dilute to 20mg / mL, dissolve eucatropine in dimethylformamide Diluted to 10 mM in DMSO, the above two solutions were used as stock solutions. The eucatropine stock solution was diluted to 0.2 mM with DMSO as a working solution. Prepare an acetonitrile solution containing 200 ng / mL midazolam and tolbutamide as a working stop solution. Add 300 μL of stop solution to each well of a 96-well plate pre-cooled on ice.
[0137] In the stability test, the polypeptide and eucatropine were respectively injected into the plasma, mixed evenly, and 100 μL of each mixed solution was transferre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com