Ophthalmic composition for treating neovascularization in posterior chamber of the eye and use thereof
An ophthalmic composition and neovascularization technology, which is applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as elevated intraocular pressure, and achieve the treatment of neovascularization in the posterior chamber of the eye proliferative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: composition and preparation method thereof
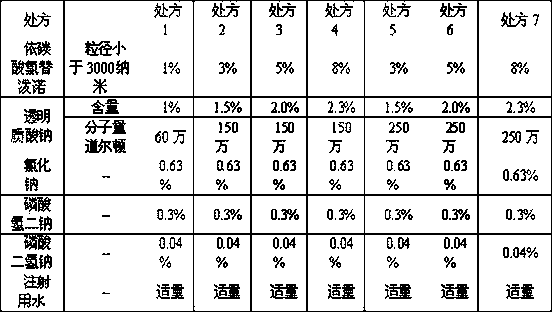
[0032] Table 1, ophthalmic composition formula
[0033]
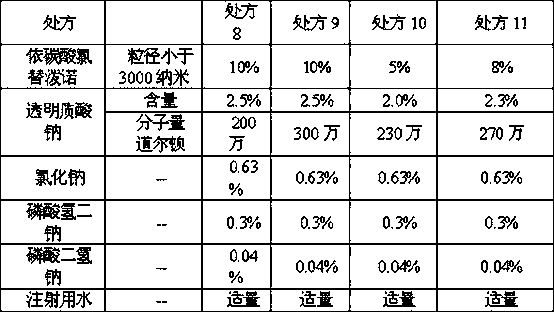
[0034] Table 2, ophthalmic composition formula
[0035]
[0036] Preparation:
[0037] 1. Micronized loteprednol etabonate
[0038] Take 1 part of loteprednol etabonate, dissolve it in 3 parts of dimethylformamide at about 60°C, quickly heat-preserve and suction filter, pour the filtrate into distilled water below 10°C for repeated washing, and then stir vigorously at a stirring speed of 220-250 revolutions per minute, continue to stir for 30 minutes, filter, wash the microcrystals repeatedly with distilled water, and vacuum-dry at 105° C. to obtain micronized loteprednol etabonate with a particle size below 3000 nanometers.
[0039] 2. Sterilize the micronized loteprednol etabonate; the aseptic treatment can be sterilized by dry heat at 105-140°C or ethylene oxide or irradiation.
[0040] 3. Preparation of sodium hyaluronate gel: take sterile s...
Embodiment 2
[0048] Embodiment 2: Animal experiment 1
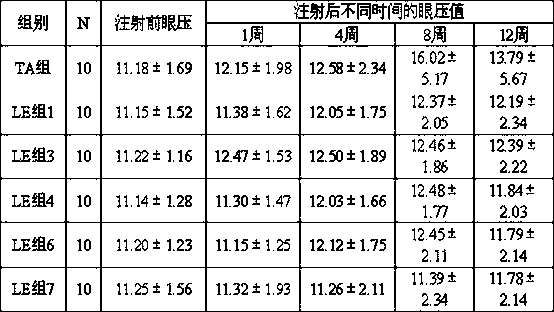
[0049]For verifying the impact of the present invention on intraocular pressure, carried out the comparative experiment to the influence of rabbit intraocular pressure, experiment is as follows:
[0050] 1. Test material
[0051] Test product: prescription 1, 3, 4, 6, 7 of embodiment 1.
[0052] Reference substance: composition and preparation method are as follows
[0053] 1) Table 3 Composition of triamcinolone acetonide gel reference substance
[0054] prescription
particle size
molecular weight
The molecular weight distribution
Dosage %
3000nm
--
--
8%
Sodium hyaluronate
--
1.5 million Daltons
1.5
2.3%
--
--
--
0.63%
--
--
--
0.3%
--
--
--
0.04%
Water for Injection
--
--
--
Appropriate amount
[005...
Embodiment 3
[0075] Embodiment 3: Animal experiment 2
[0076] The inhibitory effect of loteprednol etabonate gel on laser-induced choroidal neovascularization in rabbits is as follows.
[0077] 1. Test material
[0078] 1) Test product: prescriptions 1, 3, 4, 6, and 7 of Example 1.
[0079] 2) Experimental animals
[0080] 18 New Zealand rabbits, 8-12 weeks old, male and female, provided by Shandong Lukang Pharmaceutical Co., Ltd., license number: SCXK (Lu) 200500170, male or female, body weight 2.0-2.5kg. The feeding and use of experimental animals complied with the "Regulations on the Administration of Experimental Animals" promulgated by the National Science and Technology Committee.
[0081] Main reagents and instruments: 20% fluorescein sodium injection; 532 nm laser machine (IRIS company, USA); NF-505 fluorescein fundus angiography apparatus.
[0082] 2. Test method
[0083] 1) Animal grouping and modeling methods
[0084] 18 rabbits were divided into 6 groups, 3 in each group...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com