An activated luminescence material and a method for preparing the same
A technology of light-emitting materials and groups, applied in the field of fluorescent materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (1) The structural formula of TPE-β-CD
[0068]
[0069] (2) Preparation of TPE-β-CD inclusion compound activated luminescent material
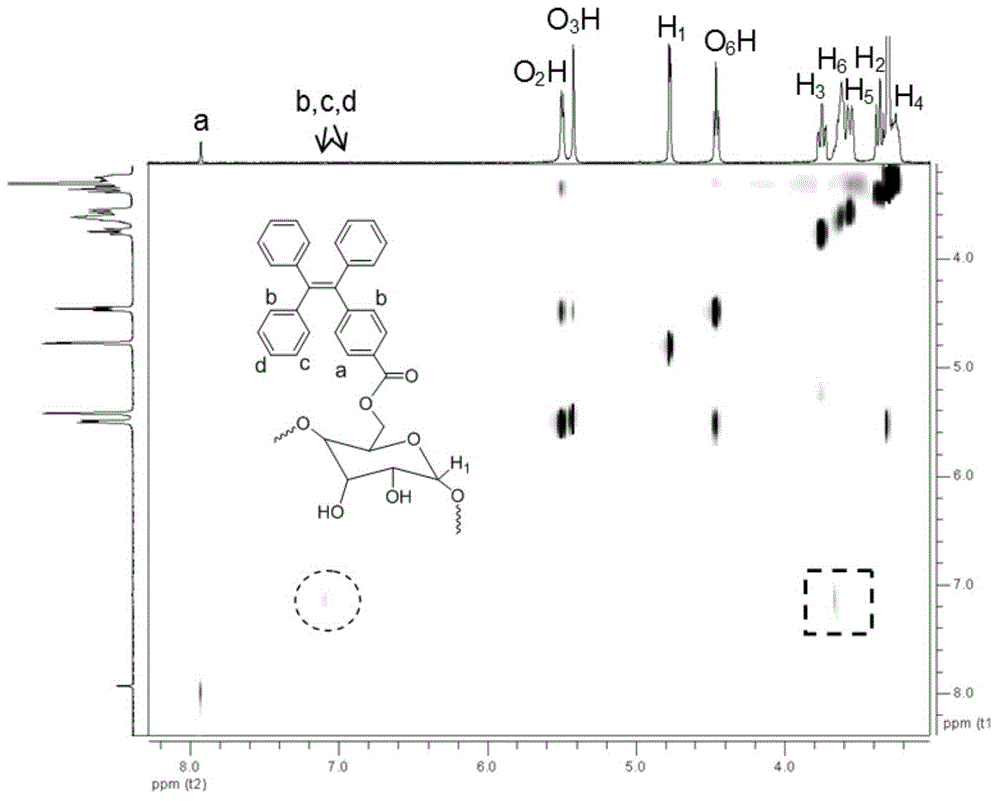
[0070] In a nitrogen atmosphere, 0.188g (0.5mmol) TPE-CO 2 H and 0.5675g (0.5mmol) β-CD were dissolved in 10ml of anhydrous dimethylformamide (DMF), and after dissolving, 0.103g (0.5mmol) of N,N-dicyclohexylcarbodiimide ( dicyclohexylcarbodiimide, DCC) in 5ml of anhydrous DMF; in a nitrogen environment, stirred at room temperature for 3 days; filtered, the filtrate was added dropwise to a large amount of diethyl ether under vigorous stirring, and a solid was precipitated; then The solid was separated by filtration again, and the solid was dried under vacuum at 40° C. overnight, with a yield of 70%. 1 H NMR (400MHz, DMSO-d 6 ), δ(TMS,ppm):3.28(d,7H,H 4 ),3.33(d,7H,H 2 ),3.55(d,7H,H 5 ),3.60(d,12H,H 6 ),3.64(br,7H,H 3 ),4.44(t,6H,O 6 H),4.80(d,7H,H 1 ),5.65(s,7H,O 3 H),5.70(d,7H,O 2 H), 7.93(s,2H,Ar-H).13 C NMR (400MHz, DM...
Embodiment 2
[0072] (1) The structural formula of TPE-α-CD:
[0073]
[0074] (2) Preparation of TPE-α-CD inclusion compound activated luminescent material
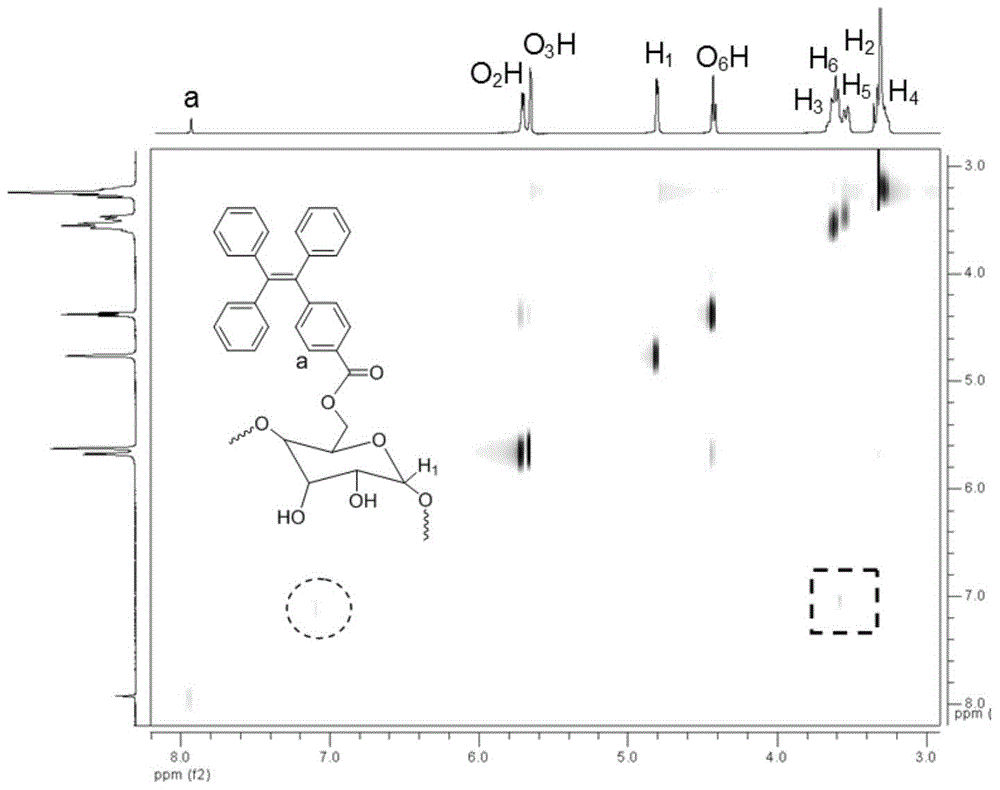
[0075] In a nitrogen atmosphere, at 0°C, 0.188g (0.5mmol) of TPE-CO 2 H and 0.486g (0.5mmol) α-CD were dissolved in 15ml of anhydrous dimethylformamide (DMF), and 0.103g (0.5mmol) of DCC was added as a catalyst; in a nitrogen environment, warm to room temperature and stir React for 3 days; filter, add the filtrate dropwise to a large amount of diethyl ether under the condition of vigorous stirring, and precipitate the solid; then filter again to obtain the solid, dry the solid overnight at 40°C under vacuum, and the yield 60%. 1 H NMR (400MHz, DMSO-d 6 ), δ(TMS,ppm):3.30(m,6H,H 4 ),3.36(m,6H,H 2 ),3.56(d,6H,H 5 ),3.61(m,10H,H 6 ),3.75(t,6H,H 3 ),4.46(t,5H,O 6 H),4.77(d,6H,H 1 ),5.41(d,6H,O 3 H),5.50(d,6H,O 2 H), 7.93(s,2H,Ar-H). 13 C NMR (400MHz, DMSO-d 6 ), δ(TMS,ppm): 59.7(C 6 ),71.8(C 2 ),72.3(C 5 ),72.9(C 3 ),...
Embodiment 3
[0077] (The structural formula of 1TPE-γ-CD:
[0078]
[0079] (2) Preparation of TPE-γ-CD inclusion compound activated luminescent material
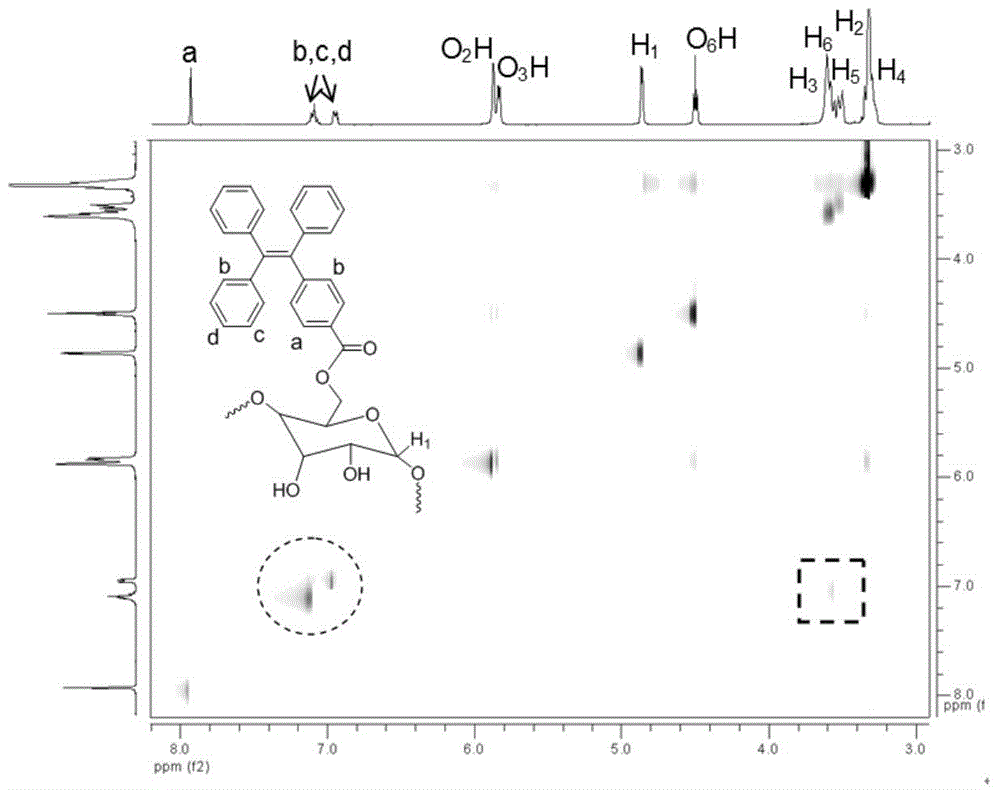
[0080] In a nitrogen atmosphere, at 0°C, 0.188g (0.5mmol) of TPE-CO 2 H, 0.648g (0.5mmol) γ-CD and 0.103g (0.5mmol) DCC were dissolved in 15ml of anhydrous dimethylformamide (DMF); in a nitrogen environment, stirred and reacted at room temperature for 3 days; Filtrate, add the filtrate dropwise to a large amount of diethyl ether under vigorous stirring, and precipitate a solid; then filter again to separate the solid, and dry the solid overnight at 40°C under vacuum, with a yield of 52%. 1 H NMR (400MHz, DMSO-d 6 ), δ(TMS,ppm):3.28(d,8H,H 4 ),3.33(d,8H,H 2 ),3.55(d,8H,H 5 ),3.60(d,16H,H 6 ),3.64(br,8H,H 3 ),4.44(t,7H,O 6 H),4.80(d,8H,H 1 ),5.65(s,8H,O 3 H),5.70(d,8H,O 2 H),6.9-7.1(m,4H,Ar-H),7.93(s,2H,Ar-H). 13 C NMR (400MHz, DMSO-d 6 ):δ(TMS,ppm):59.7(C 6 ),71.8(C 2 ),72.3(C 5 ),72.9(C 3 ), 81.3 (C 4 ), 101.7(C 1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence lifetime | aaaaa | aaaaa |
| fluorescence lifetime | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com