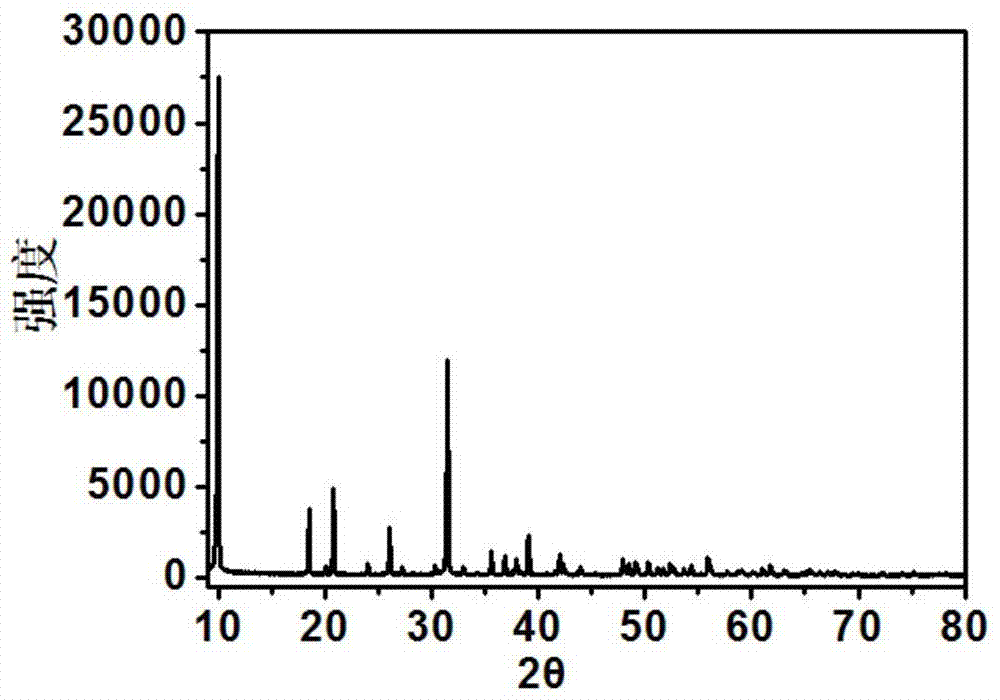
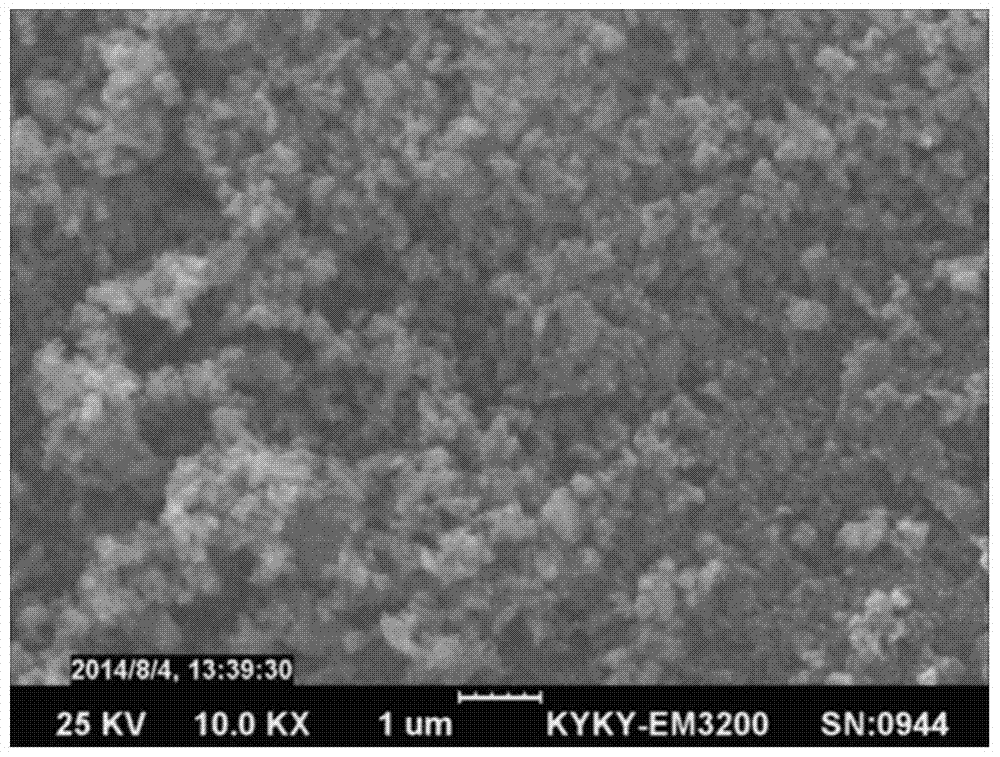
A kind of preparation method of lithium iron manganese phosphate/carbon composite material
A technology of lithium iron manganese phosphate and carbon composite materials, which is applied in the direction of electrical components, electrochemical generators, battery electrodes, etc., can solve the problems that the preparation conditions are not easy to control, and achieve easy operation control, mild reaction conditions, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add the weighed 492.34g of triammonium phosphate into a 10L reactor containing 2000g of deionized water, seal the reactor, and then turn on the stirring and nitrogen valve at a speed of 35r / min to completely dissolve the salt under stirring at room temperature , Obtaining concentration is the triammonium phosphate solution of 1.2mol / L; Ferrous sulfate 222.41g, manganese sulfate 204.87g and ascorbic acid 0.85g are dissolved in the beaker that contains 1000g deionized water, obtain molar ratio as Fe / (Fe+Mn )=0.4, the total concentration is a divalent metal salt mixed solution of 2mol / L; then at room temperature, the divalent metal salt solution is added to the reaction kettle containing the triammonium phosphate solution at a feed rate of 100r / min by a peristaltic pump . After the addition of the divalent metal salt, under the protection of nitrogen and stirring, the two solutions were allowed to continue to react at room temperature for 2 hours. After the reaction, the m...
Embodiment 2
[0044] Add the weighed 844.71g of trisodium phosphate dodecahydrate into a 10L reaction kettle containing 2000g of deionized water, seal the reaction kettle, and then open the stirring and nitrogen valve at a speed of 20r / min to make the salt stir at room temperature Dissolve completely below, obtain the triammonium phosphate solution that concentration is 1.1mol / L; Anhydrous ferrous chloride 102.42g, anhydrous manganese chloride 152.61g and ascorbic acid 1.275g are dissolved in the beaker that contains 1000g deionized water, obtain Molar ratio is that Fe / (Fe+Mn)=0.4, total concentration is the divalent metal salt mixed solution of 2mol / L; After that, the divalent metal salt solution is added to the In a reactor containing triammonium phosphate solution. After the divalent metal salt is added, under the protection of nitrogen and stirring, the two solutions are allowed to continue to react at room temperature for 4 hours. After the reaction is completed, discharge the material...
Embodiment 3
[0047] Add the weighed 471.68g of tripotassium phosphate into a 10L reactor containing 1000g of deionized water, seal the reactor, and then turn on the stirring and nitrogen valve at a speed of 30r / min to completely dissolve the salt under stirring at room temperature , to obtain a concentration of triammonium phosphate solution of 2.2mol / L; 109.11g of anhydrous ferrous nitrate, 353.54g of manganese nitrate tetrahydrate and 1.38g of ascorbic acid were dissolved in a beaker containing 2000g of deionized water to obtain the molar ratio Fe / Mn =3:7, total concentration is the divalent metal salt mixed solution of 1mol / L; After that, at room temperature, the divalent metal salt solution is added to the reaction kettle containing the triammonium phosphate solution at a feed rate of 60r / min by a peristaltic pump middle. After the divalent metal salt is added, under the protection of nitrogen and stirring, the two solutions are allowed to continue to react at room temperature for 4 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com