Invokana sustained release preparation and preparation method thereof
A slow-release preparation, the technology of canagliflozin, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of no canagliflozin sustained-release preparations, etc., and achieve The effect of being easy to industrialized production, reducing adverse reactions, and reducing the number of administrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
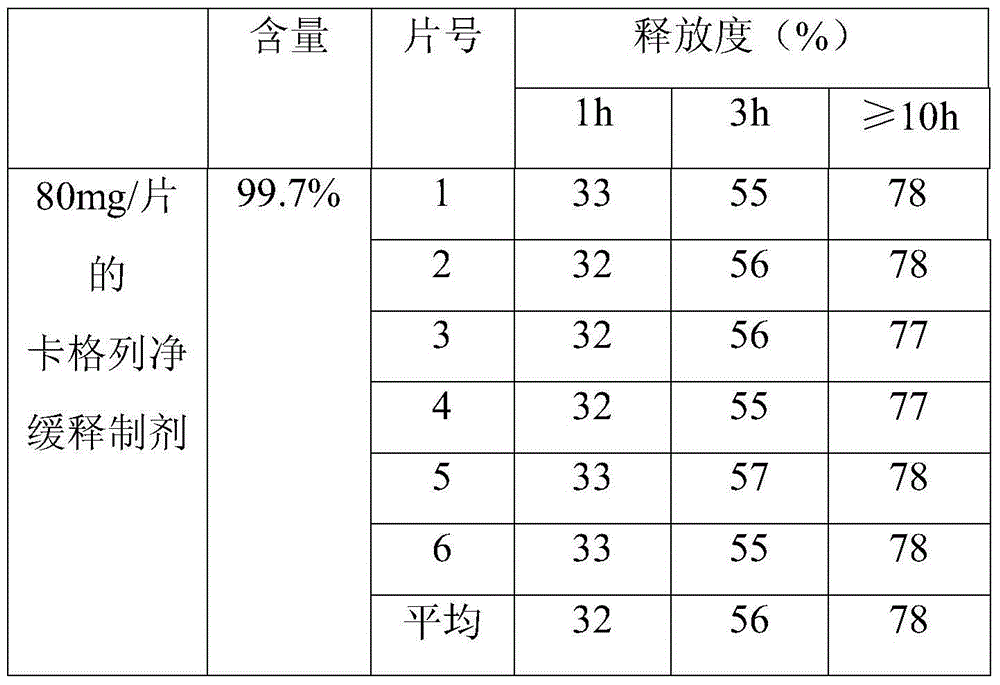
[0035] A canagliflozin sustained-release preparation, which is composed of the following raw materials:
[0036] Canagliflozin with a purity of 99.0% is 80g; polymethacrylate is 40g; sodium polymethacrylate is 20g; lactose is 50g; micronized silica gel is 10g;
[0037] First pass canagliflozin through a 100-mesh sieve, polymethacrylate, sodium polymethacrylate, lactose, and micropowdered silica gel through a 80-mesh sieve, then mix the above-mentioned raw materials, and then directly press into tablets with a hardness of 50N-80N to obtain The canagliflozin sustained-release preparation. In this embodiment, the canagliflozin sustained-release preparation was made into 80 mg / tablet.
Embodiment 2
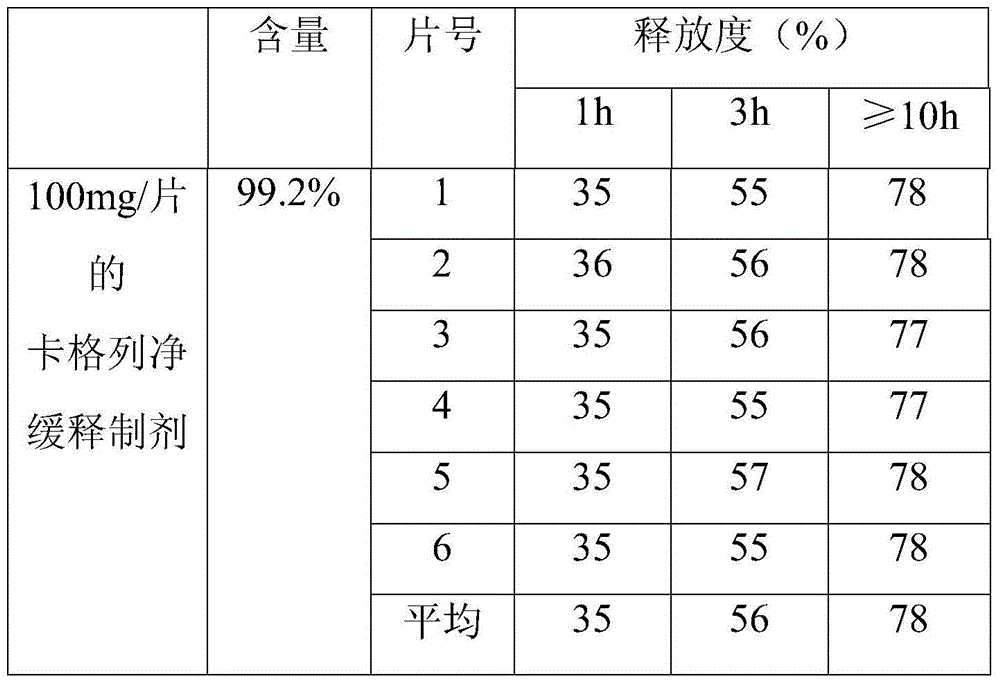
[0039] A canagliflozin sustained-release preparation, which is composed of the following raw materials:
[0040] Canagliflozin with a purity of 99.0% is 100g; polymethacrylate is 40g; sodium polymethacrylate is 20g; lactose is 30g; micronized silica gel is 10g;
[0041] First pass canagliflozin through a 100-mesh sieve, polymethacrylate, sodium polymethacrylate, lactose, and micropowdered silica gel through a 80-mesh sieve, then mix the above-mentioned raw materials, and then directly press into tablets with a hardness of 50N-80N to obtain The canagliflozin sustained-release preparation. In this embodiment, the canagliflozin sustained-release preparation was made into 0.1 g / tablet.
Embodiment 3
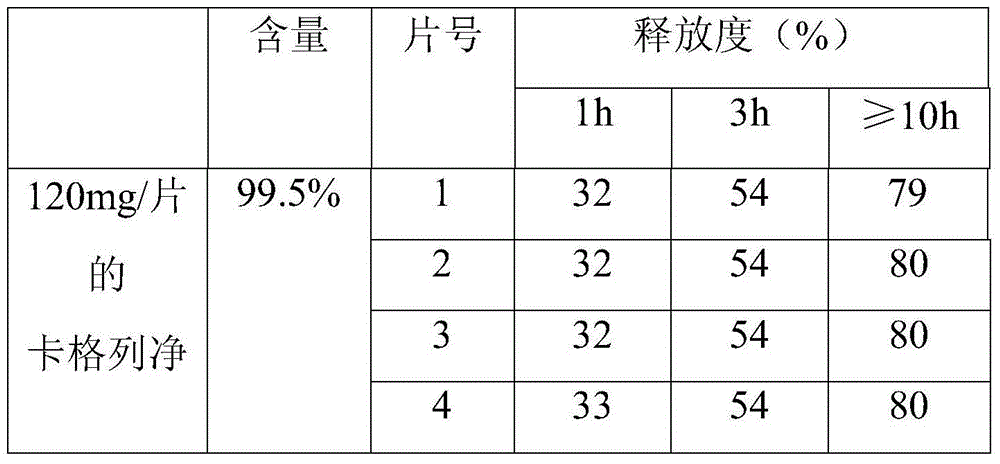
[0043] A canagliflozin sustained-release preparation, which is composed of the following raw materials:
[0044] Canagliflozin with a purity of 99.0% is 120g; polymethacrylate is 40g; sodium polymethacrylate is 10g; lactose is 30g; micronized silica gel is 10g;
[0045] First pass canagliflozin through a 100-mesh sieve, polymethacrylate, sodium polymethacrylate, lactose, and micropowdered silica gel through a 80-mesh sieve, then mix the above-mentioned raw materials, and then directly press into tablets with a hardness of 50N-80N to obtain The canagliflozin sustained-release preparation. In this embodiment, the canagliflozin sustained-release preparation was made into 0.12 g / tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com