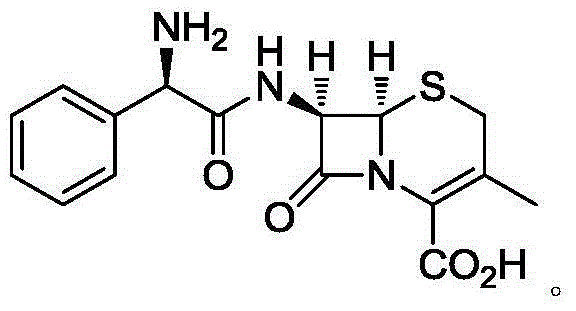
Cefradine capsule and preparation method thereof
A technology for cefradine and capsules, applied in the field of cefradine capsules and their preparation, can solve the problems of unsuitable wet granulation, unstable chemical properties, high temperature sensitivity, etc., so as to overcome poor fluidity, improve fluidity and lubricating effects , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
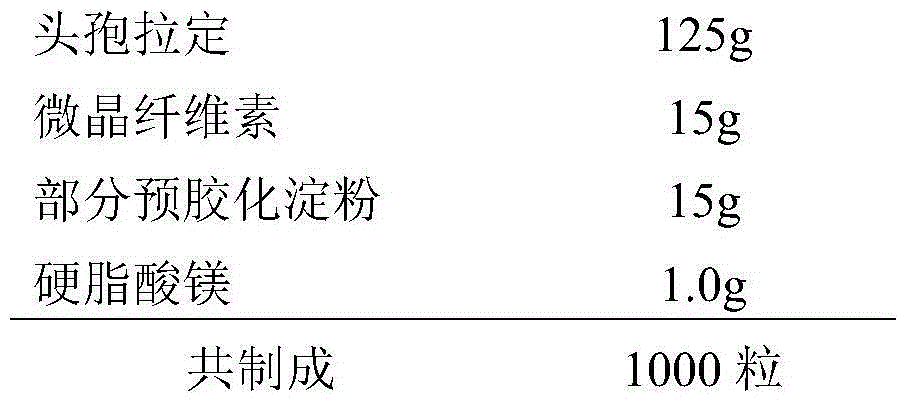
[0023] A kind of cephradine capsule, its prescription is composed as follows:
[0024]
[0025] Preparation:
[0026] (1) Drying: Dry part of the pregelatinized starch and microcrystalline cellulose in a hot air circulation oven at 100±5°C for 2 hours;
[0027] (2) sieving: cross 20 mesh sieves with cephradine and magnesium stearate, and cross 40 mesh sieves after drying microcrystalline cellulose and part of pregelatinized starch;
[0028] (3) Mixing: Weigh the powder of each raw and auxiliary material obtained in step (2) according to the prescription amount, mix cephradine, microcrystalline cellulose and part of pregelatinized starch in a mixer for 20 minutes, mix well, and then add Magnesium stearate, mixed for 5 minutes, to obtain the contents required for filling the capsule;
[0029] (4) flush filling: the content that step (3) is made is packed in the hollow capsule by filling capacity, and the difference of control filling capacity is within ± 10%, makes cephradi...
Embodiment 2
[0032] A kind of cephradine capsule, its prescription is composed as follows:
[0033]
[0034] Preparation:
[0035] (1) Drying: Dry part of the pregelatinized starch and microcrystalline cellulose in a hot air circulation oven at 100±5°C for 2 hours;
[0036] (2) sieving: cross 20 mesh sieves with cephradine and magnesium stearate, and cross 40 mesh sieves after drying microcrystalline cellulose and part of pregelatinized starch;
[0037] (3) Mixing: Weigh the powder of each raw and auxiliary material obtained in step (2) according to the prescription amount, mix cephradine, microcrystalline cellulose and part of pregelatinized starch in a mixer for 20 minutes, mix well, and then add Magnesium stearate, mixed for 5 minutes, to obtain the contents required for filling the capsule;
[0038] (4) flush filling: the content that step (3) is made is packed in the hollow capsule by filling capacity, and the difference of control filling capacity is within ± 10%, makes cephradi...
Embodiment 3
[0041] A kind of cephradine capsule, its prescription is composed as follows:
[0042]
[0043] Preparation:
[0044] (1) Drying: Dry part of the pregelatinized starch and microcrystalline cellulose in a hot air circulation oven at 100±5°C for 2 hours;
[0045] (2) sieving: cross 20 mesh sieves with cephradine and magnesium stearate, and cross 40 mesh sieves after drying microcrystalline cellulose and part of pregelatinized starch;
[0046] (3) Mixing: Weigh the powder of each raw and auxiliary material obtained in step (2) according to the prescription amount, mix cephradine, microcrystalline cellulose and part of pregelatinized starch in a mixer for 20 minutes, mix well, and then add Magnesium stearate, mixed for 5 minutes, to obtain the contents required for filling the capsule;
[0047] (4) flush filling: the content that step (3) is made is packed in the empty capsule by filling capacity, and the difference of control filling capacity is within ± 7.5%, makes cephradi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com