Macrocyclic Inhibitors of Flaviviridae Viruses
A technology of spiro rings and compounds, applied in the field of macrocyclic inhibitors of Flaviviridae viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0260] Preparation of macrocycles
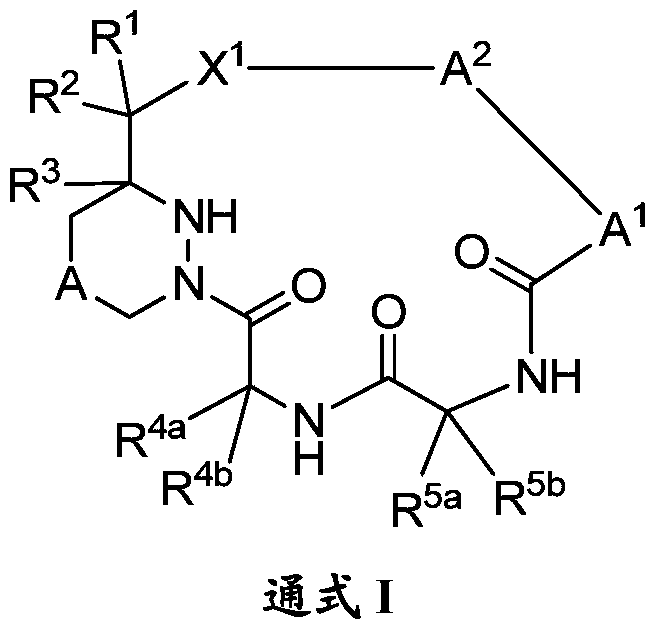
[0261] Compounds of the invention, such as those of general formula (I) and (II), can be prepared according to the schemes described below, but it will be appreciated that modifications of the illustrated methods or other methods can also be used. As illustrated in Scheme 1, from the five key components A-E, through the appropriate use of protecting groups (PG) by those skilled in the art 1 -PG 8 ), and combine them in order to synthesize the macrocyclic compound M (Q is NH). The dashed lines numbered 1-5, referred to herein as connection 1, connection 2, etc. respectively, are the 5 connections used to bind components A-E. The order in which a particular linkage occurs can vary and depends on the choice of protecting group and the desired chemistry. Typically, ligation 3, 4 or 5 is used as the final macrocyclization step.
[0262] plan 1
[0263]
[0264] Linkages 1, 2 and 3 are amide bonds. Linkages are formed between representati...
Embodiment 1 to 20
[0365] For Examples 1 to 20, unless otherwise noted, preparative HPLC was performed on a Gilson HPLC system using an Agilent Eclipse XDB / C187micron, 250 x 21.2 mm semi-preparative column and an acetonitrile / water mobile phase at a flow rate of 20 mL / min.
[0366] For Examples 121 to 175, unless otherwise noted, preparative HPLC was performed on a Shimadzu HPLC system using a 21.2 x 250 mm 10 micro C18 Phenomenex Gemini semi-preparative column and an acetonitrile / water mobile phase at a flow rate of 20 mL / min.
[0367] List of Abbreviations and Acronyms
[0368] Abbreviation Meaning
[0369] ℃ degrees Celsius
[0370] di-tBuXPhos 2-di-tert-butylphosphino-3,4,5,6-tetramethyl-2′,4′,6′-triisopropyl-1,1′-bis
[0371] benzene
[0372] 2,6-lut. 2,6-lutidine
[0373] MNBA 2-methyl-6-nitrobenzoic anhydride
[0374] 4AMS 4 angstrom molecular sieve
[0375] Acetyl
[0376] ACN acetonitrile
[0377] app obvious
[0378] Aq water content
[0379] BINAP (2,2'-bis(biphenylphosphino...
Embodiment 1
[0503] Example 1: (E)-(2R,5S,11S,14S,17R,18R)-18-hydroxy-14-isopropyl-2,11,17-trimethyl-3,9,12,15, 28-Pentaza-tricyclo[21.3.1.1*5,9*]octadeca-1(26),21,23(27),24-tetraen-4,10,13,16-tetraone : Compound 1
[0504]
[0505] Preparation of 1-((1R,5S)-10,10-dimethyl-3,3-dioxo-3λ*6*-thio-4-aza-tricyclo[5.2.1.0* in toluene (50 mL) A solution of 1,5*]dec-4-yl)-propan-1-one (3.95 g, 14.55 mmol) was then evaporated to dryness. This process was repeated, and the resulting white solid was dissolved in anhydrous dichloromethane (16 mL). A small amount of calcium hydride was added, followed by tert-butyldimethylsilyl trifluoromethanesulfonate (3.83 mL, 14.5 mmol) and anhydrous triethylamine (2.33 mL, 16.7 mmol). The reaction mixture was stirred at RT ("RT") for 15 hours ("h") under a nitrogen atmosphere. The resulting solution was evaporated to give a thick paste which was redissolved in anhydrous dichloromethane (15 mL) and added dropwise to anhydrous dichloromethane (20 mL ) in a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com